Abstract
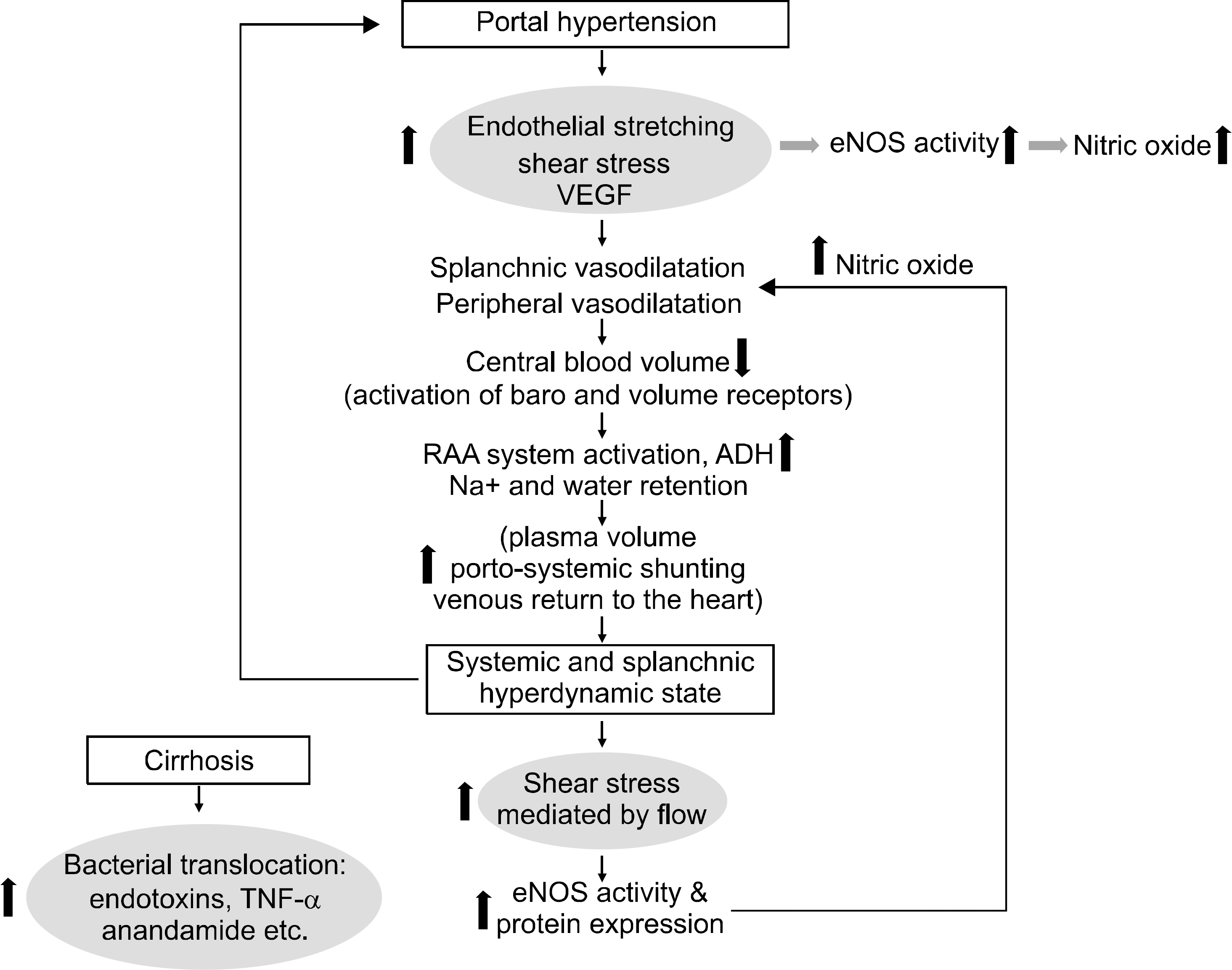
Portal hypertension (PHT) is associated with changes in the intrahepatic, systemic and portosystemic collateral circulations. Alteration in vasoreactivity (vasodilation and vasoconstriction) plays a central role in the pathogenesis of PHT by contributing to increased intrahepatic resistance, hyperdynamic circulation and the expansion of the collateral circulation. PHT is also importantly characterized by changes in vascular structure; termed vascular remodeling, which is an adaptive response of the vessel wall that occurs in response to chronic changes in the environment such as shear stress. Angiogenesis, the sprouting of new blood vessels, also occurs in PHT, especially in the expansion of the portosystemic collateral circulation. These complementary processes of vasoreactivity, vascular remodeling and angiogenesis represent important targets in the research for the treatment of portal hypertension.
REFERENCES
1. Baik SK. Assessment and current treatment of portal hypertension. Korean J Hepatol. 2005; 11:211–217.
3. Baik SK, Jeong PH, Ji SW, et al. Acute hemodynamic effects of octreotide and terlipressin in patients with cirrhosis: a randomized comparison. Am J Gastroenterol. 2005; 100:631–635.

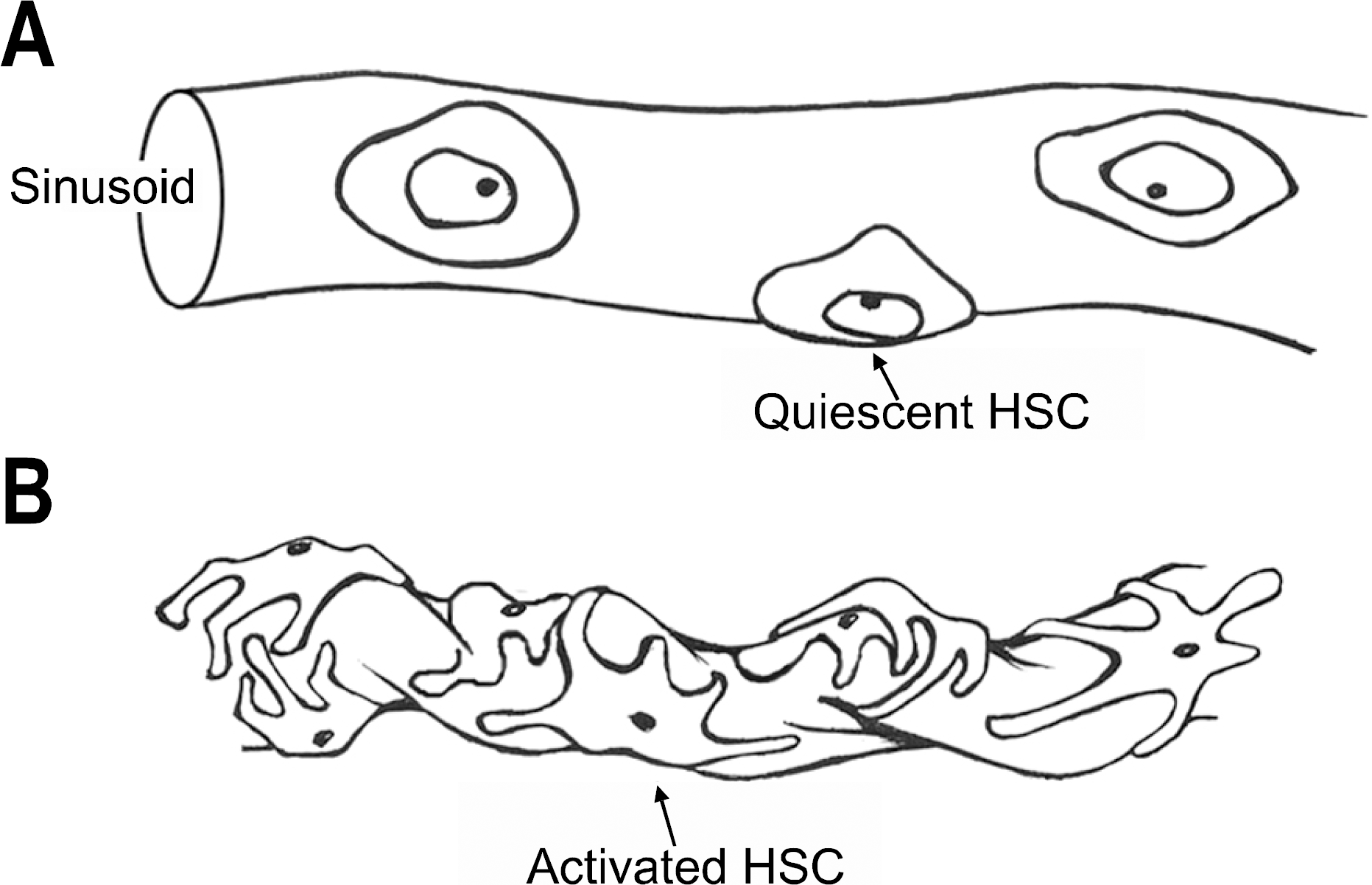
4. Lee JS, Semela D, Iredale J, Shah VH. Sinusoidal remodeling and angiogenesis: a new function for the liver-specific peri-cyte? Hepatology. 2007; 45:817–825.

5. Rockey DC. Hepatic blood flow regulation by stellate cells in normal and injured liver. Semin Liver Dis. 2001; 21:337–349.

6. Reynaert H, Thompson MG, Thomas T, Geerts A. Hepatic stellate cells: role in microcirculation and pathophysiology of portal hypertension. Gut. 2002; 50:571–581.

7. Baik SK, Park DH, Kim MY, et al. Captopril reduces portal pressure effectively in portal hypertensive patients with low portal venous velocity. J Gastroenterol. 2003; 38:1150–1154.

8. Ferná ndez-Varo G, Ros J, Morales-Ruiz M, et al. Nitric oxide synthase 3-dependent vascular remodeling and circulatory dysfunction in cirrhosis. Am J Pathol. 2003; 162:1985–1993.
10. Baik SK. Pharmacological therapy of portal hypertension–focused on Korean data. Korean J Gastroenterol. 2005; 45:381386.
11. Bhathal PS, Grossman HJ. Reduction of the increased portal vascular resistance of the isolated perfused cirrhotic rat liver by vasodilators. J Hepatol. 1985; 1:325–337.

12. Park DH, Baik SK, Choi YH, et al. Inhibitory effect of angiotensin blockade on hepatic fibrosis in common bile duct-li-gated rats. Korean J Hepatol. 2007; 13:61–69.
13. Rockey D. The cellular pathogenesis of portal hypertension: stellate cell contractility, endothelin, and nitric oxide. Hepatology. 1997; 25:2–5.

14. Reinehr RM, Kubitz R, Peters-Regehr T, Bode JG, Hä ussinger D. Activation of rat hepatic stellate cells in culture is associated with increased sensitivity to endothelin 1. Hepatology. 1998; 28:1566–1577.

15. Shah V, Toruner M, Haddad F, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999; 117:1222–1228.

16. Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998; 114:344–351.

17. Gupta TK, Toruner M, Chung MK, Groszmann RJ. Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats. Hepatology. 1998; 28:926–931.

18. Yokomori H, Oda M, Yoshimura K, et al. Elevated expression of caveolin-1 at protein and mRNA level in human cirrhotic liver: relation with nitric oxide. J Gastroenterol. 2003; 38:854–860.

19. Morales-Ruiz M, Cejudo-Martí n P, Ferná ndez-Varo G, et al. Transduction of the liver with activated Akt normalizes portal pressure in cirrhotic rats. Gastroenterology. 2003; 125:522–531.

20. Liu S, Premont RT, Kontos CD, Zhu S, Rockey DC. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat Med. 2005; 11:952–958.

21. Abraldes JG, Rodriguez-Vilarrupla A, Graupera M, et al. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. J Hepatol. 2007; 46:1040–1046.

22. Zafra C, Abraldes JG, Turnes J, et al. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004; 126:749–755.

23. Friedman SL, Rockey DC, McGuire RF, Maher JJ, Boyles JK, Yamasaki G. Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology. 1992; 15:234–243.

24. Lee JS, Kang Decker N, Chatterjee S, Yao J, Friedman S, Shah V. Mechanisms of nitric oxide interplay with Rho GTPase family members in modulation of actin membrane dynamics in pericytes and fibroblasts. Am J Pathol. 2005; 166:1861–1870.

25. Daniels CE, Wilkes MC, Edens M, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004; 114:1308–1316.
26. Ceni E, Crabb DW, Foschi M, et al. Acetaldehyde inhibits PPARgamma via H2O2-mediated c-Abl activation in human hepatic stellate cells. Gastroenterology. 2006; 131:1235–1252.
27. Aleffi S, Petrai I, Bertolani C, et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005; 42:1339–1348.

28. Hellströ m M, Kalé n M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999; 126:3047–3055.
29. Langer DA, Shah VH. Nitric oxide and portal hypertension: interface of vasoreactivity and angiogenesis. J Hepatol. 2006; 44:209–216.

30. Wiest R, Shah V, Sessa WC, Groszmann RJ. NO over-production by eNOS precedes hyperdynamic splanchnic circulation in portal hypertensive rats. Am J Physiol. 1999; 276:G1043–1051.
31. Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006; 43(2 suppl 1):S121–S131.

32. Wiest R, Das S, Cadelina G, Garcia-Tsao G, Milstien S, Groszmann RJ. Bacterial translocation in cirrhotic rats stim-ulates eNOS-derived NO production and impairs mesenteric vascular contractility. J Clin Invest. 1999; 104:1223–1233.

33. Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothe-lium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998; 101:731–736.

34. Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996; 87:1153–1155.

35. Fernandez M, Mejias M, Angermayr B, Garcia-Pagan JC, Rodés J, Bosch J. Inhibition of VEGF receptor-2 decreases the development of hyperdynamic splanchnic circulation and portal-systemic collateral vessels in portal hypertensive rats. J Hepatol. 2005; 43:98–103.

36. Fernandez M, Vizzutti F, Garcia-Pagan JC, Rodes J, Bosch J. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology. 2004; 126:886–894.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download