Abstract
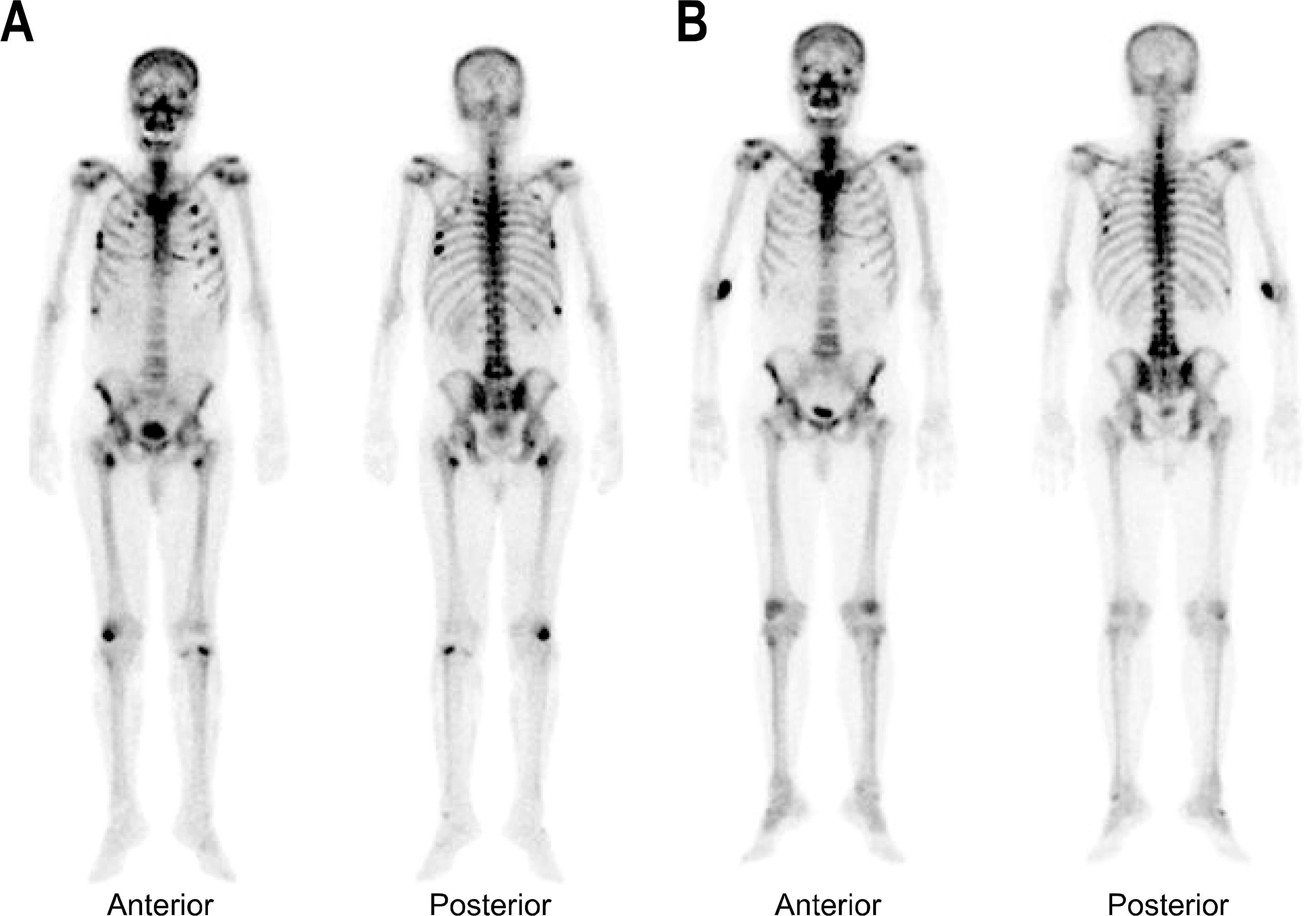
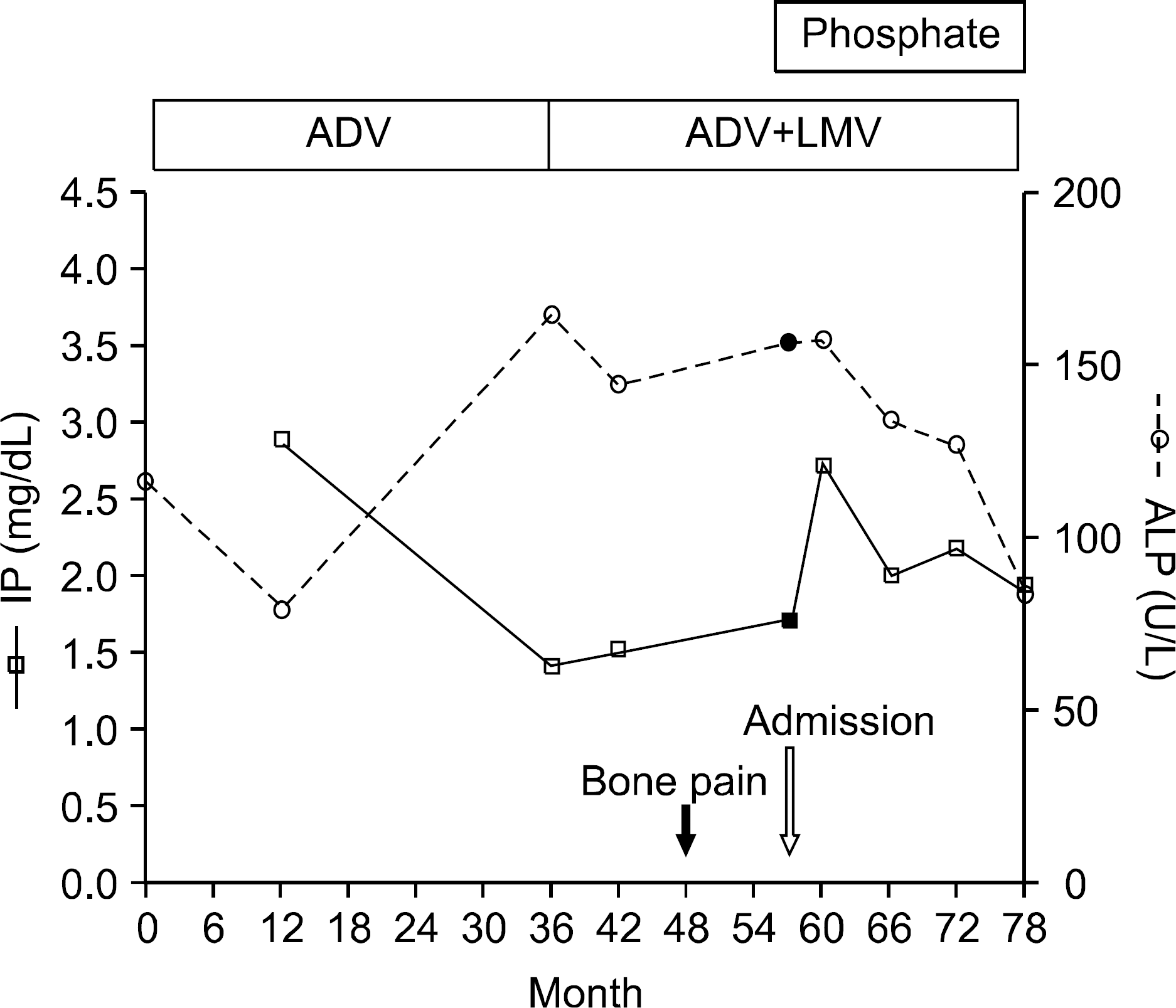
Adefovir dipivoxil, an acyclic nucleoside analogue, has been approved for the treatment of patients with chronic hepatitis B. This agent is efficacious particularly in those who have developed lamivudine resistance. The report according to hypophosphatemia induced by low dose adefovir therapy is very rare. We report one case in which osteomalacia with hypophosphatemia developed in a patient with chronic hepatitis B on adefovir dipivoxil at a low dose, 10 mg daily. A 66-year-old man, who had been taking adefovir for more than 4 years due to lamivudine resistance, presented with muscle weakness and bone pain in both thighs. After 3 years of adefovir therapy, hypophosphatemia and elevated serum alkaline phosphatase levels had been noted. A bone scan showed multiple hot uptakes. All the image findings and clinical symptoms, such as bone pain and muscle weakness were improved after correcting the hypophosphatemia with oral phosphorous supplementation.
REFERENCES
1. Lee KS, Kim DJ; Guideline Committee of the Korean Association for the Study of the Liver. Management of chronic hepatitis B. Korean J Hepatol. 2007; 13:447–488.
2. Tanji N, Tanji K, Kambham N, Markowitz GS, Bell A, D'agati VD. Adefovir nephrotoxicity: possible role of mitochondrial DNA depletion. Hum Pathol. 2001; 32:734–740.

3. Lee HC, Song YD, Ahn KJ, et al. A case of adult onset hy-pophosphatemic osteomalacia. J Korean Soc Endocrinol. 1991; 6:75–81.
4. Izzedine H, Hulot JS, Launay-Vacher V, et al. Renal safety of adefovir dipivoxil in patients with chronic hepatitis B: two double-blind, randomized, placebo-controlled studies. Kidney Int. 2004; 66:1153–1158.

5. Kramata P. Votruba I, Otová B, Holý A. Different inhibitory potencies of acyclic phosphonomethoxyalkyl nucleotide ana-logs toward DNA polymerase alpha, delta and epsilon. Mol Pharmacol. 1996; 49:1005–1011.
6. Fisher EJ, Chaloner K, Cohn DL, et al. The safety and efficacy of adefovir dipivoxil in patients with advanced HIV disease: a randomized, placebo-controlled trial. AIDS. 2001; 15:1695–1700.

7. Kahn J, Lagakos S, Wulfsohn M, et al. Efficacy and safety of adefovir dipivoxil with antiretroviral therapy: a randomized controlled trial. JAMA. 1999; 282:2305–2312.
8. Benhanmou Y, Bochet M, Thibault V, et al. Safety and efficacy of adefovir dipivoxil in patients co-infected with HIV-1 and lamivudine-resistant hepatitis B virus: an open-label pilot study. Lancet. 2001; 358:718–723.
9. Hannon H, Bagnis CI, Benhamou Y, et al. The renal toler-ance of low-dose adefovir dipivoxil by lamivudine-resistant individuals co-infected with hepatitis B and HIV. Nephrol Dial Transplant. 2004; 19:386–390.

10. Izzedine H, Launay-Vacher V, Deray G. Antiviral durg-induced nephrotoxicity. Am J Kidney Dis. 2005; 45:804–817.
11. Shaw JP, Louie MS, Kirshnamurthy W, et al. Pharmacokinetics and metabolism of selected prodrugs of PMEA in rats. Drug Metab Dispos. 1997; 25:362–366.
12. Curdy KC, Barditch-Crovo P, Walker RE, et al. Clinical pharmacokinetics of adefovir in human inmmunodeficiency virus type 1-infected patients. Antimicrob Agents Chemother. 1995; 39:2401–2405.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download