Abstract
Background/Aims
Our clinical experience and recent published literatures suggest that Clostridium difficile colitis (CDC) has become more common and potentially more pathogenic in recent years. The aim of study was to evaluate changes in the epidemiological features of CDC in hospitalized patients in Korea.
Methods
We retro-spectively reviewed all patients of CDC diagnosed at Kangnam St. Mary Hospital from 1998 to 2007. CDC was defined as having a positive C. difficile cytotoxicity assay, or endoscopic or pathologic evidence of CDC.
Results
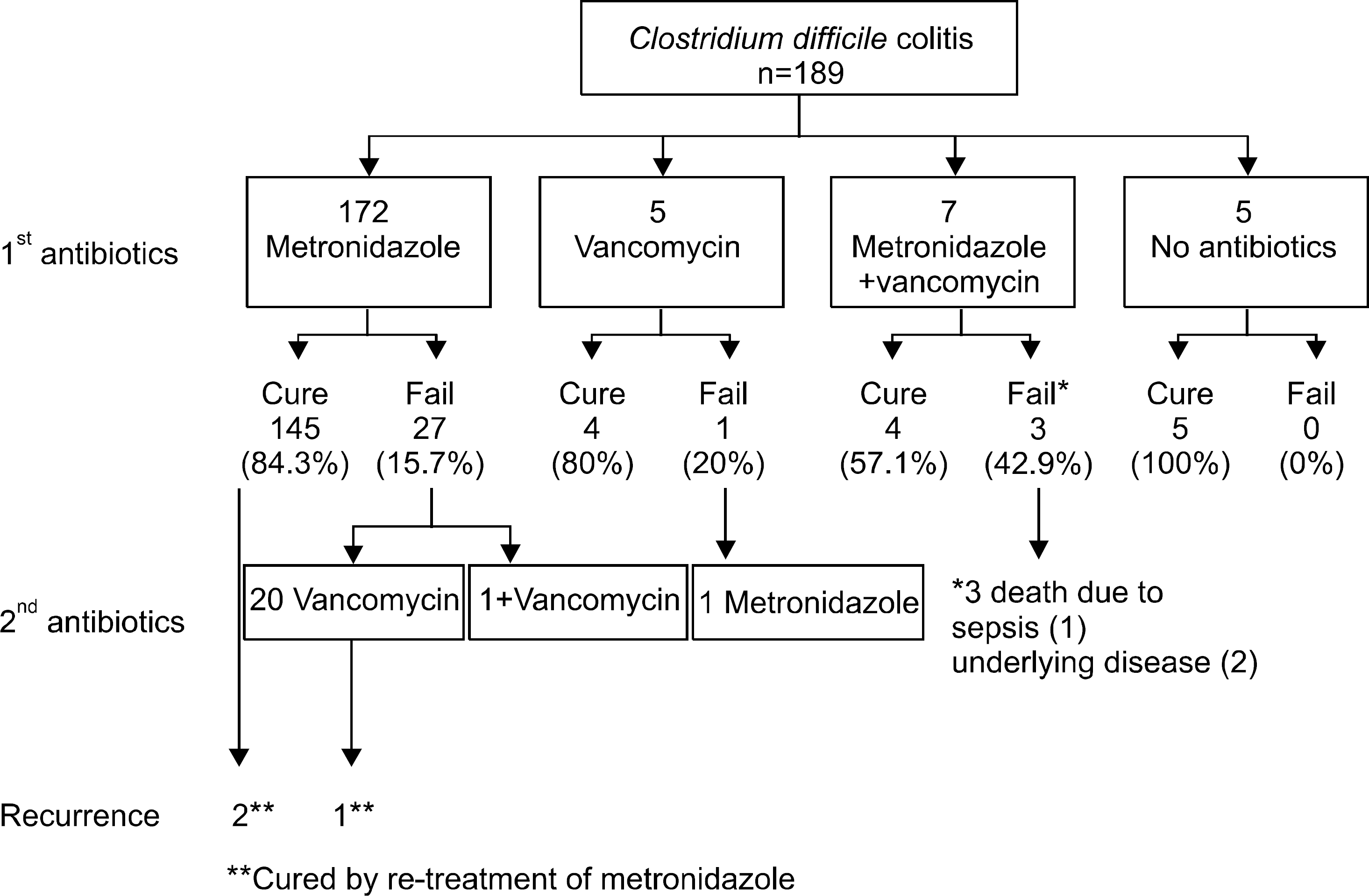
A total of 189 cases (male 73, female 116, mean age 63.3 years) of CDC were diagnosed during the study period. The prevalence of CDC increased from 1.9/10,000 patient admissions in 1998-1999 to 8.82/10,000 patient admissions in 2006-2007. One hundred sixty three indication for cases (86.2%) of patients identified a prior use of antibiotics in the 2 months preceding diagnosis. The most common antibiotic use was prophylactic use during perioperational period (33.3%) followed by pneumonia (23.3%). The overall response rate to initial antibiotics was 82.7%. One hundred seventy two (91%) patients were initially treated with metronidazole. The response rate was 84.3%. All patients with initial failure to metronidazole were successfully treated by vancomycin. The response rate of vancomycin as first treatment was 80%. Three deaths were associated with CDC despite the use of com-bination of metronidazole and vancomycin.
Go to : 
REFERENCES
1. Larson HE, Price AB, Honour P, Borriello SP. Clostridium difficile and the aetiology of pseudomembranous colitis. 1978; 1:1063–1066.
2. Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005; 353:2442–2249.
3. Pé pin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005; 173:1037–1042.
4. Pé pin J, Valiquette L, Alary ME, et al. Clostridium difficile- associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004; 171:466–472.
5. Lim PS, Yun HD, Yim KW, Song IS, Choi KW, Kim CY. A clinical study on pseudomembranous colitis. Korean J Gastrointest Endosc. 1987; 7:13–17.
6. Chung MH, Hyun MS, Lee HJ, Chung MK, Shim MC, Lee TS. A clinical study of pseudomembranous enterocolitis. Korean J Med. 1988; 34:673–680.
7. Lee CR, Lee JK, Cho YS, Yoo HM, Kim WH, Lee KW. A clinical investigation of Clostridium difficile-associated disease. Korean J Gastroenterol. 1999; 33:338–347.
8. Kim DS, Kim NS, Lee SH. Determination of defined daily dose of medicines using nominal group technique and analysis of antibiotics use in national insurance claim data: focused on antibiotics without DDD of WHO. Korean J Clin Pharm. 2007; 17:19–32.
9. Hirschborn LR, Trnka Y, Onderdonk A, Lee ML, Platt R. Epidemiology of community-aquired Clostridium difficile-associated diarrhea. J Infect Dis. 1994; 169:127–133.
10. Frost F, Hurley JS, Petersen HV, Casciano RN. Estimated incidence of Clostridium difficile infection. Emerg Infect Dis. 1999; 5:303–304.
11. Karlströ m O, Fryklund B, Tullus K, Burman LG. A prospective nationwide study of C. difficile-associated diarrhea in Sweden. The Swedish C. difficile study Group. Clin Infect Dis. 1998; 26:141–145.
12. Zilberberg MD, Shorr AF, Kollef MH. Increase in adult Clostridium difficile-related hospitalizations and case-fatality rate, United States, 2000-2005. Emerg Infect Dis. 2008; 14:929–931.
13. Barbut F, Gariazzo B, Bonné L, et al. Clinical features of Clostridium difficile-associated infections and molecular characterization of strains: results of a retrospective study, 2000-2004. Infect Control Hosp Epidemiol. 2007; 28:131–139.
14. Hubert B, Loo VG, Bourgault AM, et al. Portrait of the geo-graphic dissemination of the Clostridium difficile North American pulsed-field type 1 strain and the epidemiology of C. difficile-associated disease in Qué bec. Clin Infect Dis. 2007; 44:238–244.
15. Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005; 366:1079–1084.
16. Tae CH, Jung SA, Song HJ, et al. The first case of anti-biotic-associated colitis by Clostridium difficile PCR ribotype 027 in Korea. J Korean Med Sci. 2009; 24:520–524.
17. Shin BM, Kuak EY, Yoo HM, et al. Multicentre study of the prevalence of toxigenic Clostridium difficile in Korea: results of a retrospective study 2000-2005. J Med Microbiol. 2008; 57:697–701.
18. Abrahamian FM, Talan DA, Moran GJ, Pinner R. Update on emerging infections from the Centers for Disease Control and Prevention. Severe Clostridium difficile-associated disease in populations previously at low risk-four states, 2005. Ann Emerg Med. 2006; 48:55–59.
19. Rodriguez-Palacios A, Stä mpfli HR, Duffield T, et al. Clostridium difficile PCR ribotypes in calves, Canada. Emerg Infect Dis. 2006; 12:1730–1736.
20. Kazakova SV, Ware K, Baughman B, et al. A hospital outbreak of diarrhea due to an emerging epidemic strain of Clostridium difficile. Arch Intern Med. 2006; 166:2518–2524.
21. Muto CA, Pokrywka M, Shutt K, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005; 26:273–280.
22. Aseeri M, Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for Clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008; 103:2308–2013.
23. Monaghan T, Boswell T, Mahida YR. Recent advances in Clostridium difficile-associated disease. Gut. 2008; 57:850–860.
24. Gerding DN, Muto CA, Owens RC Jr. Treatment of Clostridium difficile infection. Clin Infect Dis. 2008; 46(suppl 1):S32–S42.
25. Pé pin J, Alary ME, Valiquette L, et al. Increasing risk of re-lapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005; 40:1591–1597.
Go to : 
Table 1.
The First Antibiotics Associated with C. difficile Colitis
Table 2.
Annual Antibiotics Usage




 PDF
PDF ePub
ePub Citation
Citation Print
Print


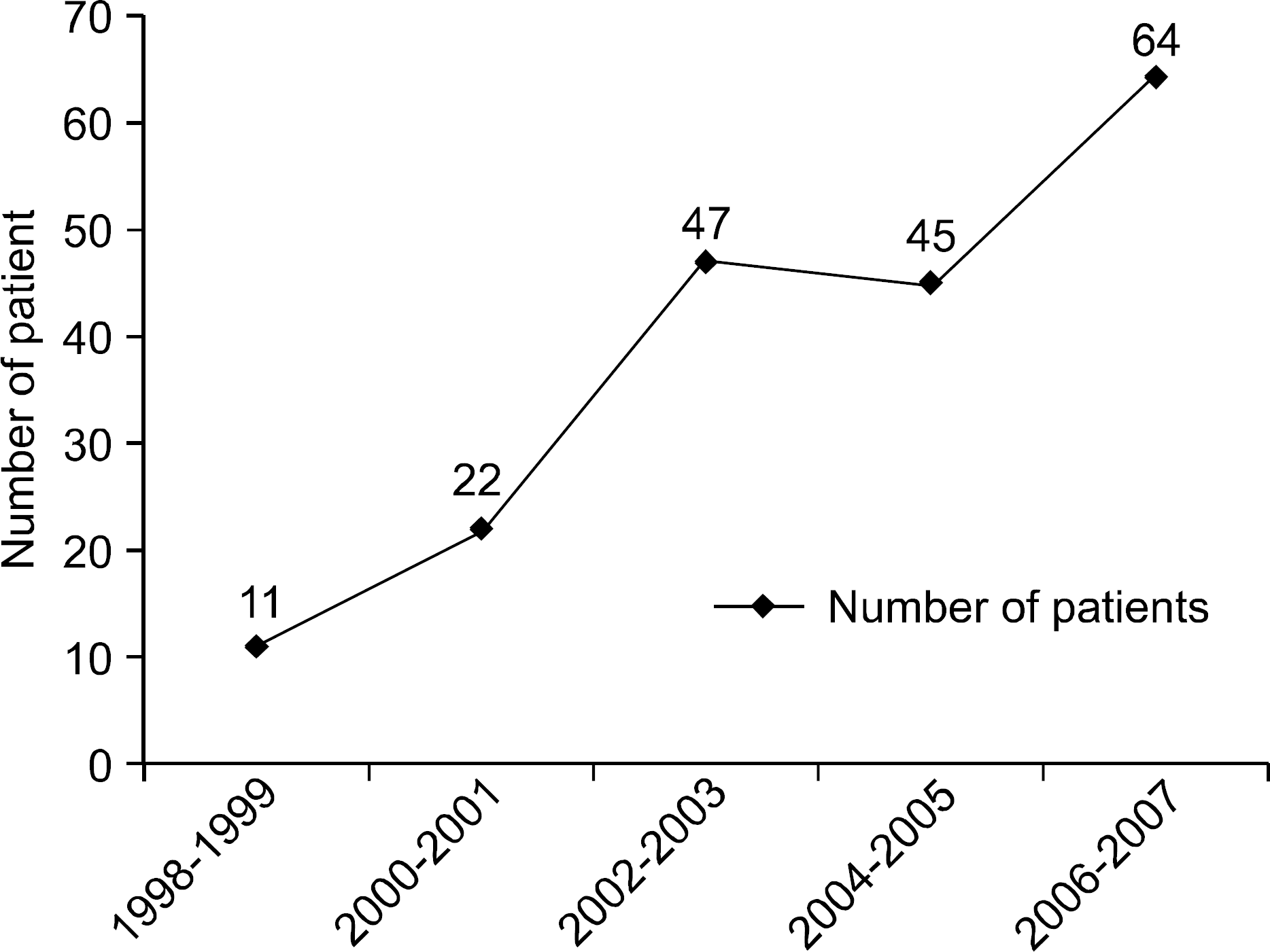
 XML Download
XML Download