Abstract
Objective
Recently it has been proposed that stem cells may be associated with the pathogenesis of endometriosis. The purposes of this study are to investigate whether the eutopic endometrial cells of women with or without endometriosis show the characteristics of stem cells in vitro and have a difference of the expressions of the undifferentiated stem cell markers as OCT-4 and CXCR4.
Methods
A total of 6 women with advanced endometriosis and a total of 10 women without endometriosis, adenomyosis or leiomyoma were included in this study. The eutopic endometrial cells, which were obtained from the menstrual blood at menstrual cycle day 2 to 4, were cultured in vitro for approximately 2 weeks, subsequently the putative very small stem cells were separated by Percoll density gradient method and were cultured. The expressions of OCT-4 and CXCR4 were analyzed by real time RT-PCR.
Results
The eutopic endometrial cells of the group of endometriosis compared with the control group showed the different morphological characteristics in vitro; more commonly heterogeneous supportive cells, very small round cells less than 3 µm and 5~15 µm sized hyperchromatic round cells. After the separation of very small round cells by Percoll density gradient method, these cells showed the several characteristics of stem cells; self-renewal, asymmetric cell division, colony formation and embryoid body-like formation. Also These cells showed the similar characteristics of very small embryonic-like stem cells; the mobile cells smaller than erythrocyte, the cell migration or adhesion to supportive cells, the sphere formation by cell aggregation and the formation of new differentiated cell by cell fusion. The expressions of OCT-4 and CXCR4 in the group of endometriosis are respectively 5.66 times and 17.69 times as high as the control group (P<0.05).
Conclusion
The very small round cells less than 3 µm and 5~15 µm sized hyperchromatic round cells, which showed the several characteristics of stem cells in vitro, were more common in eutopic endometrial cells of patients with endometriosis and the expressions of OCT-4 and CXCR4 were significantly higher. This study suggests that stem cells might play a key role in the pathogenesis of endometriosis and OCT-4 and CXCR4 might be used as a tool for diagnosis or follow-up.
Figures and Tables
 | Figure 1The epithelial cells (A) and stromal cells (B) in the control group.
Most of epithelial cells and stromal cells in the control group show homogeneous pattern. The colonies (arrow), which are composed of approximately 5~15 µm hyperchromatic round cells and very small round cells less than 3 µm, are rarely as compared with the group of endometriosis (scale bar: 50 µm).
|
 | Figure 2The epithelial cells (A) and stromal cells (B) in the group of endometriosis.
The supportive cells in the group of endometriosis show heterogeneous pattern. Very small round cells (arrow) and the colonies such as Figure 1-A are more common in the group of endometriosis compared with the control group. Very small round cells show the similar characteristics of very small embryonic-like stem cells; the mobile cells smaller than erythrocyte, the cell migration or adhesion to supportive cells, the cell aggregation (arrow) and sphere formation (scale bar: 50 µm).
|
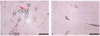 | Figure 3The asymmetric cell division of sphere-shaped cells derived from very small round cells.
Very small round cells form the sphere-shaped cells (arrow) by cell aggregation. These sphere-shaped cells adhere to other site for the undifferentiated cells (A) or adhere to for the differentiated cell by cell fusion and nuclear recombination (B) (scale bar: 50 µm).
|
 | Figure 4Colony formation (A) and embryoid body-like formation (B).
These phenomena are the morphological characteristics of stem cells in vitro culture (scale bar: 100 µm).
|
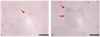 | Figure 5Self renewal (A) and embryoid body-like formation (B) of very small round cells.
At day 7 in vitro culture, the very small round cells separated by Percoll density gradient method are confluent on petri dish. Thereafter these cells differentiate gradually larger cells by cell fusion (filled arrow) or form embryoid body-like formation (open arrow) (scale bar: 50 µm).
|
 | Figure 6Asymmetric cell division (A) and neuron-like cells (B).
The sphere-shaped cells derived from very small round cells show frequently asymmetric cell division (arrow). Especially neuron-like cells are often detected (scale bar: 50 µm).
|
References
1. Speroff L, Fritz MA. Clinical gynecologic endocrinology and infertility. 2005. 7th ed. Philadelphia: Lippincott Willians & Wilkins.
2. Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am. 2003. 30:1–19. vii.

3. Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008. 1127:106–115.

4. Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927. 14:422–469.

5. Gruenwald P. Origin of endometriosis from the mesenchyme of coelomic walls. Am J Obstet Gynecol. 1942. 44:470–474.
6. Von Recklinghausen F. Adenomyomas and cystadenomas of the wall of the uterus and tube: their origin as remnants of the Wolffian body. Wien Klin Wochenschr. 1896. 8:530.
7. Sampson JA. Metastatic or embolic endometriosis, due to the mestrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927. 3:109.
9. Leyendecker G, Herbertz M, Kunz G, Mall G. Endometriosis results from the dislocation of basal endometrium. Hum Reprod. 2002. 17:2725–2736.

10. Wu Y, Strawn E, Basir Z, Wang Y, Halverson G, Jailwala P, et al. Genomic alterations in ectopic and eutopic endometria of women with endometriosis. Gynecol Obstet Invest. 2006. 62:148–159.

11. Zaehres H, Lensch MW, Daheron L, Stewart SA, Itskovitz-Eldor J, Daley GQ. High-efficiency RNA interference in human embryonic stem cells. Stem Cells. 2005. 23:299–305.

12. Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006. 20:1915–1924.

13. Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008. 267:226–244.

14. Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009. 80:1136–1145.
15. Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007. 5:57.

16. Medina MG, Lebovic DI. Endometriosis-associated nerve fibers and pain. Acta Obstet Gynecol Scand. 2009. 88:968–975.

17. Van Kaam KJ, Schouten JP, Nap AW, Dunselman GA, Groothuis PG. Fibromuscular differentiation in deeply infiltrating endometriosis is a reaction of resident fibroblasts to the presence of ectopic endometrium. Hum Reprod. 2008. 23:2692–2700.

18. Osuga Y, Koga K, Tsutsumi O, Igarashi T, Okagaki R, Takai Y, et al. Stem cell factor (SCF) concentrations in peritoneal fluid of women with or without endometriosis. Am J Reprod Immunol. 2000. 44:231–235.

19. Starzinski-Powitz A, Zeitvogel A, Schreiner A, Baumann R. [Endometriosis--a stem cell disease?]. Zentralbl Gynakol. 2003. 125(7-8):235–238.
20. Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006. 20:857–869.

21. Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Ratajczak J, Kucia M. Very small embryonic-like stem cells: characterization, developmental origin, and biological significance. Exp Hematol. 2008. 36:742–751.

22. Zuba-Surma EK, Kucia M, Ratajczak J, Ratajczak MZ. "Small stem cells" in adult tissues: very small embryonic-like stem cells stand up! Cytometry A. 2009. 75:4–13.

23. Virant-Klun I, Rozman P, Cvjeticanin B, Vrtacnik-Bokal E, Novakovic S, Rülicke T, et al. Parthenogenetic embryo-like structures in the human ovarian surface epithelium cell culture in postmenopausal women with no naturally present follicles and oocytes. Stem Cells Dev. 2009. 18:137–150.

24. McGuckin C, Jurga M, Ali H, Strbad M, Forraz N. Culture of embryonic-like stem cells from human umbilical cord blood and onward differentiation to neural cells in vitro. Nat Protoc. 2008. 3:1046–1055.

25. Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002. 20:249–258.

26. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods. 2001. 25:402–408.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download