Abstract
Objective
The purpose of this study was to investigate the treatment effects of a topical application of 5-aminolevulinic acid (5-ALA) for photodynamic therapy (PDT) to treat cervical cancer.
Methods
We first investigated the effects of 5-ALA cream according to application time. And to find the effective 5-ALA concentration and the distribution times in vivo, 20% 5-ALA cream was topically applied to the tumor of the nude mouse. We then observed the distribution of 5-ALA via fluorescence measurement with using a 532 nm diode laser. 25 nude mice were divided into Control, ALA, Laser, and PDT group. To evaluate the PDT effect at cancer lesion, we applied 20% 5-ALA cream to the tumor by the same method, and the PDT was done by using a 632 nm diode laser at the time of the peak level of fluorescence. We checked the changes of the volume of cancer for 30 days, and then biopsy was done.
Results
The effective post-irradiation time after topical ALA application was 9 hours. In the PDT group, 40% (4/10) of the mice showed decreased tumor size.
Conclusion
The maximum PpIX fluorescence at 9 hours after local applicationof 5-ALA cream was checked. And PDT group did not show any statistical difference than control group in the growth of tumor size than control group. However responding cases (4/10) of PDT group showed the meaningful decrease of tumor size than control group (P<0.05).
Figures and Tables
Fig. 1
The application of 5-aminolevulinic acid cream was occluded during the whole application period by covering the mouse all around with an adhesive dressing (A). The mice were given 480 J/cm2 of 632 nm diode laser on the skin overlying the tumor for 20 minutes (B). The tumor was recorded every 3 days after photodynamic therapy (PDT) (C: Before PDT, D: 9 days, E: 21 days, F: 30 days).
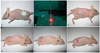
Fig. 2
The effective 5-aminolevulinic acid distribution times were measured by the degree of fluorescence of PpIX with using a 532 nm diode laser. The .most effective distribution time after ALA injection was 9 hours.

Fig. 3
Comparison of the tumor volume changes of the xenografted tumor of HeLa cancer cells in nude mouse according to four different groups. * Statistically significant difference between control and group IV respond group.

Fig. 4
These pictures shows histological changes of xenografted tumor of HeLa cervical cancer cells in nude mouse at 30 days after photodynamic therapy in Group 4. The tumor size was decreased grossly, and there are small amounts of cancer tissue microscopically (H&E stain). Group 4 (partial remission): (A) ×40, (B) ×200.

References
1. Gomer CJ, Rucker N, Ferrario A, Wong S. Properties and applications of photodynamic therapy. Radiat Res. 1989. 120:1–18.
2. Kim YW, Park CH, Ro DY, Sin JI, Bae SM, Lee JM, et al. A case of glassy cell carcinoma of the uterine cervix with photodynamic therapy prior to radical surgery. Korean J Obstet Gynecol. 2003. 46:842–846.
3. Joo SK, Ahn JC, Chung PS, Oh CH. Cell death pattern of photodynamic therapy using Hyperion and 532 nm DPSS laser in Hela cells. J Korean Photodyn Assoc. 2005. 2:33–37.
4. Daniell MD, Hill JS. A history of photodynamic therapy. Aust N Z J Surg. 1991. 61:340–348.
5. Honigsmann H, Gschnait F, Konrad K, Wolff K. Photochemotherapy for pustular psoriasis (von Zumbusch). Br J Dermatol. 1977. 97:119–126.
6. Kick G, Messer G, Plewig G. Historical development of photodynamic therapy. Hautarzt. 1996. 47:644–649.
7. Lee C, Kim J, Jeong CH, Na YJ, Kim IH, Lee SY, et al. Photodynamic therapy in the management of cervical intraepithelial neoplasia. Korean J Gynecol Oncol Colposc. 2004. 15:85–91.
8. Stender IM, Borgbjerg FM, Villumsen J, Lock-Andersen J, Wulf HC. Pain induced by photodynamic therapy of warts. Photodermatol Photoimmunol Photomed. 2006. 22:304–309.
9. Robinson DJ, de Bruijn HS, de Wolf WJ, Sterenborg HJ, Star WM. Topical 5-aminolevulinic acid-photodynamic therapy of hairless mouse skin using two-fold illumination schemes: PpIX fluorescence kinetics, photobleaching and biological effect. Photochem Photobiol. 2000. 72:794–802.
10. Ackroyd R, Kelty C, Brown N, Reed M. The history of photodetection and photodynamic therapy. Photochem Photobiol. 2001. 74:656–669.
11. Kato H. History of photodynamic therapy: past, present and future. Gan To Kagaku Ryoho. 1996. 23:8–15.
12. Wei W, Shen K. Photodynamic therapy and its application in gynecologic oncology. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2003. 25:484–486.
13. Dougherty TJ, Kaufman JE, Goldfarb A, Weishaupt KR, Boyle D, Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978. 38:2628–2635.
14. Ahn TG, Kang MC, Park JS, Cho YS, Choi BC, Kim DW, et al. A case of treatment using high-dose Megestrol Acetate (Megace(R)) combined with PDT (Photodynamic Therapy) on early stage of endometrial carcinoma, in nulliparous woman. Korean J Obstet Gynecol. 2005. 48:1023–1028.
15. Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst. 1998. 90:889–905.
16. Hopper C. Photodynamic therapy: a clinical reality in the treatment of cancer. Lancet Oncol. 2000. 1:212–219.
17. Collins Y, Einstein MH, Gostout BS, Herzog TJ, Massad LS, Rader JS, et al. Cervical cancer prevention in the era of prophylactic vaccines: a preview for gynecologic oncologists. Gynecol Oncol. 2006. 102:552–562.
18. Richart RM. Causes and management of cervical intraepithelial neoplasia. Cancer. 1987. 60:1951–1959.
19. Simon P, Dupond I. Screening for cervical cancer. Rev Med Brux. 2006. 27:S218–S220.
20. Barnett AA, Haller JC, Cairnduff F, Lane G, Brown SB, Roberts DJ. A randomised, double-blind, placebo-controlled trial of photodynamic therapy using 5-aminolaevulinic acid for the treatment of cervical intraepithelial neoplasia. Int J Cancer. 2003. 103:829–832.
21. Bodner K, Bodner-Adler B, Wierrani F, Kubin A, Szolts-Szolts J, Spangler B, et al. Cold-knife conization versus photodynamic therapy with topical 5-aminolevulinic acid (5-ALA) in cervical intraepithelial neoplasia (CIN) II with associated human papillomavirus infection: a comparison of preliminary results. Anticancer Res. 2003. 23:1785–1788.
22. Jones JM, Sweetnam P, Hibbard BM. The outcome of pregnancy after cone biopsy of the cervix: a case-control study. Br J Obstet Gynaecol. 1979. 86:913–916.
23. Leiman G, Harrison NA, Rubin A. Pregnancy following conization of the cervix: complications related to cone size. Am J Obstet Gynecol. 1980. 136:14–18.
24. Cotter A, Gardeil F, Varley M, Skinner J, Turner MJ. Are the immediate complications of conisation with the large loop similar to those with the knife? J Obstet Gynaecol. 2000. 20:63–64.
25. Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006. 367:489–498.
26. Yang JX, Shen K, Lang JH, Huang HF, Wu M, Pan LY. Cervical carcinoma in situ: a clinical and pathological analysis of 118 cases. Zhonghua Yi Xue Za Zhi. 2006. 86:300–302.
27. Andikyan V, Kronschnabl M, Hillemanns M, Wang X, Stepp H, Hillemanns P. Fluorescence diagnosis with 5-ALA thermogel of cervical intraepithelial neoplasia. Gynakol Geburtshilfliche Rundsch. 2004. 44:31–37.
28. Andrejevic-Blant S, Major A, Ludicke F, Ballini JP, Wagnieres G, van den Bergh H, et al. Time-dependent hexaminolaevulinate induced protoporphyrin IX distribution after topical application in patients with cervical intraepithelial neoplasia: A fluorescence microscopy study. Lasers Surg Med. 2004. 35:276–283.
29. Hillemanns P, Korell M, Schmitt-Sody M, Baumgartner R, Beyer W, Kimmig R, et al. Photodynamic therapy in women with cervical intraepithelial neoplasia using topically applied 5-aminolevulinic acid. Int J Cancer. 1999. 81:34–38.
30. Wierrani F, Kubin A, Jindra R, Henry M, Gharehbaghi K, Grin W, et al. 5-aminolevulinic acid-mediated photodynamic therapy of intraepithelial neoplasia and human papillomavirus of the uterine cervix-a new experimental approach. Cancer Detect Prev. 1999. 23:351–355.
31. Allison RR, Cuenca A, Downie GH, Randall ME, Bagnato VS, Sibata CH. PD/PDT for gynecological disease. Photodiagnosis Photodyn Ther. 2005. 2:51–63.
32. Ahn WS, Bae SM, Huh SW, Lee JM, Namkoong SE, Han SJ, et al. Necrosis-like death with plasma membrane damage against cervical cancer cells by photodynamic therapy. Int J Gynecol Cancer. 2004. 14:475–482.
33. Harrod-Kim P. Tumor ablation with photodynamic therapy: introduction to mechanism and clinical applications. J Vasc Interv Radiol. 2006. 17:1441–1448.
34. Lee WY, Park JH, Kim BS, Han MJ, Hahn BS. Chlorophyll derivatives (CpD) extracted from silk worm excreta are specifically cytotoxic to tumor cells in vitro. Yonsei Med J. 1990. 31:225–233.
35. Muller S, Walt H, Dobler-Girdziunaite D, Fiedler D, Haller U. Enhanced photodynamic effects using fractionated laser light. J Photochem Photobiol B. 1998. 42:67–70.
36. Peng Q, Warloe T, Berg K, Moan J, Kongshaug M, Giercksky KE, et al. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer. 1997. 79:2282–2308.
37. Untch M, Korell M, Kirschstein M, Hepp H. The synergistic effect of delta-aminolevulinic acid and photodynamic laser therapy based on an in vitro model of the ATP tumor chemosensitivity test. Gynakol Geburtshilfliche Rundsch. 1995. 35:85–89.
38. Regula J, MacRobert AJ, Gorchein A, Buonaccorsi GA, Thorpe SM, Spencer GM, et al. Photosensitisation and photodynamic therapy of oesophageal, duodenal, and colorectal tumours using 5 aminolaevulinic acid induced protoporphyrin IX-a pilot study. Gut. 1995. 36:67–75.
39. Keefe KA, Tadir Y, Tromberg B, Berns M, Osann K, Hashad R, et al. Photodynamic therapy of high-grade cervical intraepithelial neoplasia with 5-aminolevulinic acid. Lasers Surg Med. 2002. 31:289–293.
40. Pahernik SA, Botzlar A, Hillemanns P, Dellian M, Kirschstein M, Abels C, et al. Pharmacokinetics and selectivity of aminolevulinic acid-induced porphyrin synthesis in patients with cervical intra-epithelial neoplasia. Int J Cancer. 1998. 78:310–314.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download