Abstract
Purpose
To analyze the differences of semen parameters in Korean young population for three periods from 2002 to 2013.
Materials and Methods
A total of 516 semen samples were collected from Korean men presenting for infertility, varicoceles or other infectious problems for three periods from 2002 to 2012: January 2002-December 2003, January 2007-December 2008, and January 2012-December 2013. A standard World Health Organization procedure for semen analysis was performed for assessment of semen concentration, volume, motility, morphology, and pH.
Results
A total of 160, 162, 194 men constituted the study populations in 2002 to 2003, in 2007 to 2008, and in 2012 to 2013, respectively. The overall sperm parameter results suggested a statistically significant difference between 2002 to 2003 and 2012 to 2013 except pH. However, considering the data from 2007 to 2008, there were no trends in changes in overall semen parameters. Negative correlations were observed in all semen parameters with increasing age in all patients, except for pH. In addition, semen volume, motility, and morphology had higher negative correlation coefficients with age, from 2002 to 2013, serially.
Semen analysis is one of the first steps in approaching to infertile male patients. Since 1987, the World Health Organization (WHO) has published the "WHO Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction", and the fifth edition manual was released in 2010. Based on a population study of fertile men across 14 countries, the 2010 criteria included lower reference values than the 1999 manual [1]. Considering that the reference values were created from a distribution of fertile men whose partners had a time-to-pregnancy of 12 months or less, it can be inferred that the past reference values were too high or that average semen parameters declined. Over the last several decades, there have been a large number of debates about the issue of declining semen parameters. In 1974, declining semen parameters were first reported by Nelson and Bunge [2], and it was thought because of environmental changes. Since then, many studies have been published try to find a correlation between semen parameters and environment. In 1992, Carlsen et al. [3] conducted a study of definite decline in semen parameters over 50 years. In that analysis, the authors found that semen density had decreased at a rate of -0.93×106/mL/y (from 113×106/mL to 66×106/mL) [3].
Many other studies have reported a similar decline. However, Swan et al. [4] reported significant differences in semen density for non-Western countries from that of the U.S. and European. In addition, Becker and Berhane [5] reported a significant decline was found only in the U.S. studies. Eventually, studies from specific region have found a significant decrease in semen density, but worldwide decrease has not been shown.
Very few studies have been reported in non-Western countries, especially in Korea. Recently, Seo at al. [6] carried out a study in Korea to evaluate trends in semen analysis for 10 years in 22,249 men attending their clinics in 1999. However, the authors did not find any decline in semen concentration. Trends in changes in semen parameter are still debated. Therefore in this paper we provide the trends in the overall changes of semen quality of Korean men attending National Police Hospital for three periods from 2002 to 2013. This retrospective chart review study emphasized not only changes in semen parameters but also age specific changes.
A total of 516 men constituted the study population for three periods from 2002 to 2013: 160 men from January 2002 to December 2003, 162 men from January 2007 to December 2008 and 194 men from January 2012 to December 2013. They visited our institution for infertility work-ups, varicoceles or other infectious problems (orchitis, chronic prostatitis, etc.). The diagnoses of patients were as follows: 25 infertilities, 62 varicoceles, and 73 other infectious diseases in 2002-2003, 32 infertilities, 74 varicoceles, 56 other infectious diseases in 2007-2008, 54 infertilities, 94 varicoceles, 46 other infectious diseases in 2012-2013. Records of patients were obtained from our hospital information system and the data were analyzed with approval of our institution review board (IRB No.: 11100177-201509-HR-008).
Each man masturbated into a sterile wide-mouthed plastic container after a 3-day abstinence from ejaculation. The semen samples were collected only once per person. All semen samples liquefied at 37℃ for 20 minutes before semen analysis. An experienced technician performed manual semen analysis for the semen parameters: pH, semen volume, concentration, motility, and morphology. The semen samples from 2002 to 2003 and from 2007 to 2008 were analyzed according to WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction, 4th edition [7]. In addition, the semen samples from 2012 to 2013 were analyzed according to WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction, 5th edition [8]. All of the semen samples were analyzed in a single testing laboratory of our institution. Extreme pathological disorders were excluded by selecting only semen concentration >20×106/mL. Samples with major liquefaction were also excluded.
Statistical analysis of the data was calculated using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA) and Microsoft Excel 2010 (Microsoft, Redmond, WA, USA). The distributions of the semen parameters were calculated separately from 2002 to 2013 and were found to be approximately normal. Hence, differences between semen parameters from 2002 to 2013 were analyzed by one-way analysis of variances (ANOVA) and Tukey test for multiple comparisons, p<0.05 was considered statistically significant. In addition, the Pearson correlation coefficient was used to examine the relationship between the semen parameters and age from 2002 to 2013.
A total of 160, 162, and 194 men constitute the study populations in 2002-2003, in 2007-2008, and in 2012-2013, respectively. The mean ages in the study populations were 25.48±8.12, 26.24±7.45, and 30.30±9.31 in 2002, 2007, and 2012, respectively (p<0.05). The overall sperm parameter results are presented in Fig. 1 and Table 1. In this paper, it suggested that there has been a significant decline in semen concentration, motility, morphology, and volume between 2002 to 2003 and 2012 to 2013. (74.68×106/mL, 61.04×106/mL; 64.74%, 54.77%; 80.10%, 78.22%; 4.41 mL, 2.93 mL) However, when we analyzed all data including 2007 to 2008 by ANOVA and multiple comparison test, semen concentration and morphology had no statistically significant differences between 2002 to 2003, and 2007 to 2008. Moreover there was a significant increase in semen motility between 2002 to 2003, and 2007 to 2008. In addition semen concentration, volume and pH had no statistically significant differences between 2007 to 2008 and 2012 to 2013.
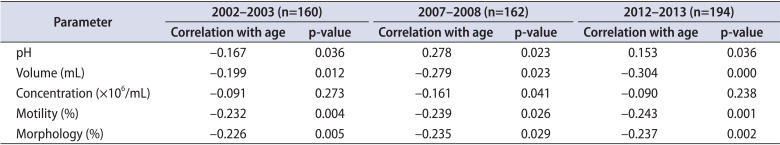
Pearson correlation coefficients were examined to study the degree of the relationship between the semen parameters and age in 2002, 2007, and 2012. The correlation coefficients and p-values are shown in Table 2. Negative correlations were found in all semen parameters with increasing age in all patients, except for pH. Moreover, semen volume and morphology had higher negative correlation coefficients with age, from 2002 to 2013, serially.
Many studies on semen analysis suffered from many limitations, such as selection bias, confounding factors, small study populations, and regional and seasonal differences. Although we made careful efforts to reduce these defects in the current study, a problem in relation to the selection of study populations remained. At first the chief complaints of our study population were not homogenous. They visited our center for infertility work-ups, varicoceles, or other infectious problems (orchitis, chronic prostatitis, etc.). Furthermore the mean ages in the study populations were different from one another in 2002, 2007, and 2012. If we had separated the study population into small groups, the small study population could have come into question. Therefore, even though we excluded extreme pathological disorders by selecting only a semen concentration >20×106/mL, surely it cannot be said that the current study reflects those of the normal population. In addition, regional characteristics, such as urban or rural area, were not considered. These were difficult points to interpret in the results in comparison to other studies. Ideally, a population-based study would be suitable for reflecting the general population. However, it seems very difficult to conduct a population-based study in this field. Thus, we were reminded that it was difficult to generalize the results to the general population. On the other hand, the 5th WHO manual for semen analysis was published in 2010. One of significant change from 4th editions was the categorization of sperm motility. In 5th WHO manual, sperm motility was divided into progressively motile, nonprogressively motile and immotile. Moreover in semen morphology, sperm with a normal morphology of 4% was contrast to sperm with a normal morphology of 14% in the 4th edition [7,8,9]. However in our study, those changes were not significantly concerned. Therefore it could be significant limitation in our paper. At last, we thought about seasonal variation in semen parameters. In one study, Chen et al. [10] reported significant seasonal differences in some semen parameters. The mean sperm concentration and morphology were the highest in winter, on the other hand semen volume, pH were similar. However we collected semen parameters over two years, seasonal variation could be ignorable.
As mentioned, numerous studies have reported the decline of semen parameters. On the other hand, trends in the changes in semen parameters are still debated. However, our study suggested a significant decrease in semen concentration, motility and morphology between 2002 to 2003 and 2012 to 2013. Even though, by multiple comparison test of three periods, semen concentration, motility and morphology had no specific trends from 2002 to 2013 in our study. Declining semen parameters were thought to be due to environmental changes. Many endocrine-disrupting chemicals that are likely to act as estrogens or androgens could be one of possible reasons behind decreasing semen parameters [11]. In addition, environmental pollutants, such as PCB (polychlorinated biphenyl), DDT (dichloro-diphenyltrichloroethane), Dieldrin and Toxaphene are not easily degraded, and their accumulation remains in soil or water. These substances are known to create abnormalities in humans and wild animals [12]. However in most countries the use of those environmental pollutants has already been prohibited from the 1980s. Nevertheless, in our study, a significant decrease in semen concentration, motility and morphology from 2002 to 2012 was observed. Recently, many studies were conducted on the effect of other types of environmental pollutants. For example, phthalates in a various plastic objects have been found to impair spermatogenesis. Furthermore, workers who were unduly exposed to heavy metals, such as cadmium, chromium, lead, manganese and mercury have decreased semen concentration, volume, and motility [13]. Some of these studies implied the direct effect of environmental pollutant on semen parameters, while others showed damage at the genetic level without changes in semen parameters. As it were, a negative influence of environmental pollutant on semen parameter and destruction at the genetic level were reported [14,15].
In addition, Mukhopadhyay et al. [16] reported a decline in semen parameters in Kolkata, India by comparing those of a study population from 1981 to 1985 with those of a study population from 2000 to 2006. In their study, the concentration of air pollutants, such as suspended particulate matter, respirable particulate matter and NOx increased in the same period according to the data of the National Environmental Engineering Research Institute in Kolkata, India [16]. On the one hand, to investigate air pollution, PM10 (particulate matter less than 10 µm) was measured from 1995 in Korea. According to the Korean National Institute of Environmental Research, 65,100 tons, 98,143 tons, and 119,980 tons of PM10 was reported in 2002, 2007, and 2012, respectively [17]. Therefore, one of the possible reasons behind declining semen parameters could be related with air pollutant. Certainly, a further prospective study is needed.
Interestingly, in our study, negative correlations were observed in all semen parameters with increasing age in all patients, except for pH. Moreover, semen volume (-0.199, -0.279, -0.304), motility (-0.232, -0.239, -0.243), and morphology (-0.226, -0.235, -0.237) had higher negative correlation coefficients with age, from 2002 to 2013, serially (Table 2). Recently Kidd et al. [18] reviewed the past studies on the relationship between semen parameters and age. They reported that aging process is correlated with a decrease in semen volume, morphology but not with semen concentration. However, serial changes in negative correlation coefficient between age and semen parameters were not evaluated. Therefore our results could be interpreted that the influence of environmental factors became more powerful over time. As it were, it is evident that a decrease in semen parameters with age was stronger in 2012 than in 2002 and 2007. One of the possible reasons behind declining semen parameters in the aging process is that environmental changes might have enhanced our chance of exposure to endocrine-disrupting chemicals, heavy metals, and air pollutants. Because of confounding factors in this study, a further prospective study is needed. On the other hand in this paper, mean ages in study populations were 25.48±8.12, 26.24±7.45, and 30.30±9.31 in 2002, 2007, and 2012, respectively. Mean ages were similar among studies population, therefore it could come into question to analysis age related differences of semen parameters among them. However young men in 20s or 30s are actively trying to conceive. According to Statistics Korea, the average age when Koreans married for the first time was 31.8 for men in 2014 and Korean men married 2.5 years later than in 2000 [19]. In addition, the infertile couple for the delayed marriage became a social problem in Korea. Therefore we thought that it could be some meaningful to analysis age related differences of semen among study population, even though mean ages of study population were young and similar.
Eventually in this study, there were significant changes in the semen parameters of the study populations from 2002 to 2013. Semen volume and morphology showed higher negative correlation coefficients with age, from 2002 to 2013, serially. However, as mentioned our results showed changes of the semen parameters of men visiting our institution. Therefore a well-designed prospective study should be prepared and performed to resolve the question of semen parameters in Korea.
In the present study, there were no significant changes in semen paramaters of study population from 2002 to 2013. In addition, semen volume and morphology showed higher negative correlation coefficients with age from 2002 to 2013, serially. However this study consisted of data from a single institution, there may be a potential for selection bias. Therefore, additional prospective evaluations are needed for generalizing our results.
References
1. Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010; 16:231–245. PMID: 19934213.

2. Nelson CM, Bunge RG. Semen analysis: evidence for changing parameters of male fertility potential. Fertil Steril. 1974; 25:503–507. PMID: 4835605.

3. Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992; 305:609–613. PMID: 1393072.

4. Swan SH, Elkin EP, Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environ Health Perspect. 1997; 105:1228–1232. PMID: 9370524.

5. Becker S, Berhane K. A meta-analysis of 61 sperm count studies revisited. Fertil Steril. 1997; 67:1103–1108. PMID: 9176451.

6. Seo JT, Rha KH, Park YS, Lee MS. Semen quality over a 10-year period in 22,249 men in Korea. Int J Androl. 2000; 23:194–198. PMID: 10886420.

7. World Health Organization. WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction. 4th ed. Cambridge: Cambridge University Press;1999.
8. World Health Organization. WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction. 5th ed. Cambridge: Cambridge University Press;2010.
9. Murray KS, James A, McGeady JB, Reed ML, Kuang WW, Nangia AK. The effect of the new 2010 World Health Organization criteria for semen analyses on male infertility. Fertil Steril. 2012; 98:1428–1431. PMID: 22921910.

10. Chen Z, Toth T, Godfrey-Bailey L, Mercedat N, Schiff I, Hauser R. Seasonal variation and age-related changes in human semen parameters. J Androl. 2003; 24:226–231. PMID: 12634309.

11. Baker K, Li J, Sabanegh E Jr. Analysis of semen parameters in male referrals: impact of reference limits, stratification by fertility categories, predictors of change, and comparison of normal semen parameters in subfertile couples. Fertil Steril. 2015; 103:59–65.e5. PMID: 25450301.

12. Henderson BE, Benton B, Jing J, Yu MC, Pike MC. Risk factors for cancer of the testis in young men. Int J Cancer. 1979; 23:598–602. PMID: 37169.

13. Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014; 32:1–17. PMID: 24872947.

14. De Rosa M, Zarrilli S, Paesano L, Carbone U, Boggia B, Petretta M, et al. Traffic pollutants affect fertility in men. Hum Reprod. 2003; 18:1055–1061. PMID: 12721184.
15. Selevan SG, Borkovec L, Slott VL, Zudova Z, Rubes J, Evenson DP, et al. Semen quality and reproductive health of young Czech men exposed to seasonal air pollution. Environ Health Perspect. 2000; 108:887–894. PMID: 11017895.

16. Mukhopadhyay D, Varghese AC, Pal M, Banerjee SK, Bhattacharyya AK, Sharma RK, et al. Semen quality and agespecific changes: a study between two decades on 3,729 male partners of couples with normal sperm count and attending an andrology laboratory for infertility-related problems in an Indian city. Fertil Steril. 2010; 93:2247–2254. PMID: 19328484.

17. National Institute of Environmental Research. Emission statistics [Internet]. Incheon: National Institute of Environmental Research;c2009. cited 2015 Oct 5. Available from: http://airemiss.nier.go.kr/nape/statistics/airpollution/pollutants/retrieve.jsp?mm=2&sm=1&ssm=1.
18. Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001; 75:237–248. PMID: 11172821.

19. Statistics Korea. Vital statistics [Internet]. Daejeon: Statistics Korea;2015. cited 2015 Oct 5. http://kostat.go.kr.
Fig. 1
Changes in semen parameters (A, concentration; B, motility; C, morphology; D, pH; E, volume) within three periods.
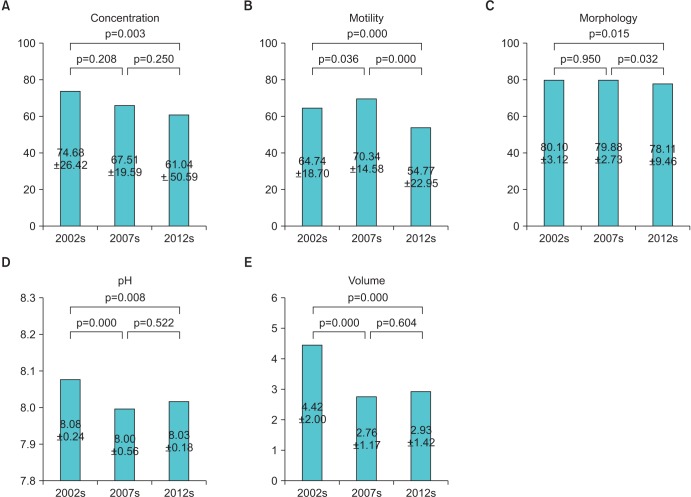
Table 1
Comparison of semen parameters from 2002 to 2013
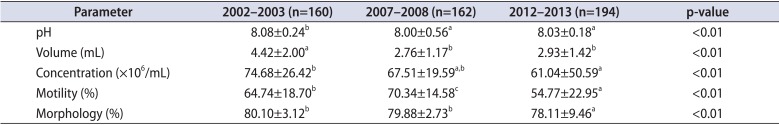
Table 2
Correlation between semen parameters and age from 2002 to 2013




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download