Abstract
Purpose
To report the initial clinical outcomes of the newly devised sliding loop technique (SLT) used for renorrhaphy in patients who underwent robot-assisted laparoscopic partial nephrectomy (RALPN) for small renal mass.
Materials and Methods
We reviewed the surgical videos and medical charts of 31 patients who had undergone RALPN with the SLT renorrhaphy performed by two surgeons (CWJ and CK) between January 2014 and October 2014. SLT renorrhaphy was performed after tumor excision and renal parenchymal defect repair. Assessed outcomes included renorrhaphy time (RT), warm ischemic time, perioperative complications, and perioperative renal function change. RT was defined as interval from the end of bed suture to the renal artery declamping.
Results
In all patients, sliding loop renorrhaphy was successfully conducted without conversions to radical nephrectomy or open approaches. Mean renorrhaphy and warm ischemic time were 9.0 and 22.6 minutes, respectively. After completing renorrhaphy, there were no adverse events such as dehiscence of approximated renal parenchyma, renal parenchymal tearing, or significant bleeding. Furthermore, no postoperative complications or significant renal function decline were observed as of the last follow-up for all patients. The limitations of this study include the small volume case series, the retrospective nature of the study, and the heterogeneity of surgeons.
Because of an increased detection rate of small renal masses (4 cm or less), partial nephrectomy (PN) as a nephron-sparing surgery (NSS) has been conducted in a number of centers as the gold standard of care [1]. In cautiously selected patients with tumor sizes of <7 cm, PN can be considered feasible with superior renal functional preservation and comparable oncologic and survival outcomes to radical nephrectomy [12]. Currently, the application of pure laparoscopic or robotic approaches for minimally invasive nephron-sparing surgery (MINSS) has emerged as an alternative modality to the existing open approach because MINSS can provide several additional advantages, such as better cosmetic results, shorter periods of convalescence, and decreased blood loss, as well as comparable oncologic and functional outcomes [34].
Despite advantages with laparoscopic partial nephrectomy (LPN) and robot-assisted laparoscopic partial nephrectomy (RALPN), renorrhaphy remains a challenge in surgery. To ensure a convenient and safe procedure, many renorrhaphy techniques have been devised and applied in clinical settings [56789101112]. Previously, we had devised the sliding loop technique (SLT) by modifying the conventional sliding clip technique introduced by Benway et al.[67], and established that SLT was feasible for renorrhaphy in a porcine model. The comparative analysis between the two techniques showed that SLT is superior to the sliding clip technique in terms of lowered risks of renal parenchymal dehiscence, as it allows more tension following renorrhaphy [13]. In this paper, based on our feasibility experiments in animal models, we attempted to verify the clinical feasibility and safety of SLT renorrhaphy in patients undergoing RALPN.
This study design and the use of patients' information stored in the hospital database were approved by the Institutional Review Board (IRB) at the Seoul National University Hospital (SNUH) (IRB No. H-1501-002-635). We were given exemption from obtaining informed consent by the IRB because the present study is a retrospective study and personal identifiers were completely removed and the data were analyzed anonymously. Our study was conducted according to the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
We collected and reviewed the electronic medical records and surgical videos of 31 patients who had undergone RALPN for small renal masses by two surgeons (CWJ and CK) at SNUH between January 2014 and October 2014. The decision to perform RALPN was mainly determined according to tumor characteristics and patient's individual preferences, so patients were not randomized based on distinct institutionalized standards or guidelines.
Under general anesthesia, a nasogastric tube and Foley catheter were inserted in the supine position. Then, the patients were turned to the flank position at approximately 60 degrees. In all cases, surgical access was achieved transperitoneally. Pneumoperitoneum was created by the use of a Veress needle. Basically, four robotic arm approaches, consisting of a 12-mm camera (30 degree downward lens) port and three robotic working ports (8 mm) were commonly used with two additional trocars for assistance, consisting of a 12-mm trocar located at the periumbilical area and a 5-mm trocar located at a point about 8 cm toward the caudal side from where camera port was used. The placement of trocars and their locations in RALPN is presented in Fig. 1.
Location of renal mass was approximately identified on the basis of preoperative imaging studies. Gerota's fascia was incised and the surrounding perirenal fat was removed to expose the renal mass. After the exact margin and depth of the tumor was confirmed with an intraoperative flexible ultrasound, the resection margin was outlined with electrocautery, with consideration for a sufficient margin of normal parenchymal tissue around the tumor.
Mannitol (0.5 mg/kg) was intravenously administered before hilar vessel clamping to prevent ischemic renal injury. After clamping of the renal artery with laparoscopic bulldog clamps, tumor excision was performed with cold scissors. The excised tumor was located above the liver or spleen for later retrieval using Endobag at the end of surgery.
After replacing the equipment of the 1st and 2nd robotic arms with robotic needle drivers, in order to ensure hemostasis and to repair any collecting system openings, bed suturing of the tumor resection site was conducted with continuous running suture using about 15 cm of 3-0 V-Loc 90 (Covidien, Dublin, Ireland) barbed suture material (Fig. 2A). Following bed suturing, biologic hemostatic tissue sealant (i.e., fibrilla) was applied to the parenchymal defect site.
The SLT used for renorrhaphy in our study was a modification of the conventional sliding clip technique [567]. All suture materials for renorrhaphy were prepared in advance on a scrub table. The basic renorrhaphy procedure was similar between the two techniques. However, the setting up of suture material and the method of creating tension are slightly different between SLT and the sliding clip technique. We designed a suture material of approximately 13 cm in length with a loop, which had a length of about 1 cm after being tied three times at the end of each 1-0 Vicryl (Ethicon Inc., Somerville, NJ, USA) thread, and placed a TFE polymer pledget (7.0 mm×3.0 mm) (Ethicon Inc.) and a 10-mm Weck Hem-O-Lok (Teleflex, Research Triangle, NC, USA) clip below the knot (Fig. 3).
Each prepared suture material was inserted through the renal capsule at intervals of approximately 1 cm, and the needle was passed through the premade loop (Fig. 2B). By pulling the thread toward the direction of needle passage with a robotic needle driver, a second Hem-O-Lok clip was placed on the thread. Then, the surgeon pushed the Hem-O-Lok clip perpendicularly toward the renal parenchyma using another robotic needle driver to tighten the renorrhaphy (Fig. 2C). The same sliding technique was performed for the remaining sutures. Additionally, LapraTy (Ethicon Inc.,) clips were placed at each thread just above the second Hem-O-Lok clip to maintain the tension of sutures (Fig. 2D).
Through the series of processes mentioned above (Fig. 2; Video clip, Supplementary material), secure tightness of renal parenchymal suturing can be obtained. Following renorrhaphy, the renal artery was unclamped by a laparoscopic bulldog remover and the tumor resection bed was examined to check for additional bleeding. The needle was cut and extracted. If needed, hemostatic agents were applied to the renorrhaphy site.
The excised tumor and perirenal fat were placed in an Endobag and extracted via the 12-mm assistance port, which may be extended in cases of larger tumors. A Jackson-Pratt drain was inserted at the perirenal area. All trocars were removed under direct laparoscopic vision, and the fascia at the 12-mm camera and 12-mm port sites was closed with a thick nonabsorbable suture material to prevent the risk of herniation.
The characteristics and perioperative outcomes of the study cohort are listed in Table 1. Mean age and body mass index (BMI) were 48.5 years (range, 31-74 years) and 25.3 kg/m2 (range, 19.9-39.2 kg/m2), respectively. Clinical tumor size ranged from 1 to 5.4 cm. A total of 16 and 15 tumors were located on right and left side, respectively. More than half of tumors (51.6%) showed an exophytic feature.
Mean operative time and console time were 163.3 minutes (range, 90-240 minutes) and 114.8 minutes (range, 55-180 minutes), respectively. Mean renorrhaphy time (RT), which was defined as the interval from the completion of bed suturing to renal artery declamping, was 9.0 minutes (range, 5-24 minutes). Warm ischemic time (WIT) showed a mean value of 22.6 minutes, ranging from 13 to 51 minutes. Mean estimated blood loss was 330.7 mL (range, 50-1,100 mL).
SLT renorrhaphy was successfully conducted in all patients without conversion to robotic radical nephrectomy or open surgery. Af ter renorrhaphy, there were no adverse events, such as dehiscence of approximated renal parenchyma, renal parenchymal tearing, and significant bleeding in any patients.
Mean preoperative serum creatinine (Cr) level and estimated glomerular filtration rate (eGFR) were 0.91 mg/dL and 87.5 mL/min/1.73 m2, respectively, while their values at 1 month postsurgery were 0.93 mg/dL and 83.9 mL/min/1.73 m2. However, there was no statistically significant perioperative change in either serum Cr (p=0.184) and eGFR (p=0.173).
There were 3 cases (9.7%) of intraoperative transfusions (Clavien-Dindo grade II) with a mean of two packed red cell blood units. However, there were no severe postoperative complications, such as delayed hemorrhage and urine leakage, as of the latest follow-up in all patients.
For the past several years, open partial nephrectomy (OPN) has been established as the preferred therapeutic modality for the management of small localized renal masses, providing comparable oncologic and survival outcomes, and superior benefit for the preservation of renal function to radical nephrectomy [1214]. Since the introduction of MINSS using pure laparoscopic [1516] or robotic approaches [17] in the urologic field, PN applying these minimally invasive techniques has been conducted by experienced surgeons in several centers and shown to have comparable outcomes to OPN with relatively better cosmetic benefits and improved convalescence [31819]. In particular, RALPN is an emerging procedure that can provide many potential advantages, such as three-dimensional images, magnified vision, prevention of hand tremor, and fully articulating instruments [1720]. Therefore, RALPN has become regarded as a viable means of surmounting the technical limitations related to LPN [2122].
Even though MINSS has its advantages, renorrhaphy remains the most challenging part of the surgery. Renorrhaphy should be performed in a time-sensitive manner due to its impact on WIT. In any case, the shortening of WIT is generally considered as a crucial factor for the preservation of renal function in the context of PN [1123]. Renal reconstruction after tumor excision in OPN has usually been performed with traditional tied-suture renorrhaphy. Under direct vision through the open approach, knot tied-suturing for renorrhaphy can be easily performed while maintaining the suture line tension with direct hand control under acceptable WIT. Under the limitation of WIT, intracoporeal knot tied-suture renorrhaphy in MINSS may be technically difficult and time-consuming even for skillful laparoscopic surgeons because it is necessary to control the needle and proceed with renorrhaphy with the dominant hand while maintaining the suture tension with the nondominant hand.
Therefore, to simplify renorrhaphy and reduce WIT in MINSS, many knotless suturing techniques for renorrhaphy have been developed and applied in clinical practice [56789101112242526]. In particular, because knotless suturing with tight tension can be accomplished using several surgical clips including LapraTy and Hem-O-Lok clips, renorrhaphy techniques using these tools have been studied by a number of investigators [567891011122526]. Orvieto et al. [26] used simplified surgical techniques, including closure of the renal defect using LapraTy clips, for 41 patients who received LPN and reported a WIT of 29.7 minutes, but there were three cases of open conversion and a complication rate of 13.2%. V-hilar suture renorrhaphy, which is a form of knotless suturing using Hem-O-Lok clips, has been applied for renal hilar tumors in RALPN and shown to provide feasible results [925]. Recently, unidirectional barbed suture material (V-Loc) has been applied to renorrhaphy in LPN and RALPN, and shown promising results in terms of reducing WIT by approximately 20% in comparison with conventional polyglactin (Vicryl) running sutures [811]. Furthermore, as demonstrated by our study, barbed suture materials can be used for intracorporeal repair of renal parenchymal defects in MINSS [10]. Wahafu et al. [12] reported the comparative results between two renorrhaphy methods, which are conventional one layer, interrupted, figure-of-eight (OLIF) suture and two layer, continuous, unknotted (TLCU) suture using Hem-O-Lok clip, in retroperitoneal LPN. It was revealed that TLCU renorrhaphy could provide better several advantages than OLIF renorrhaphy in terms of WIT, hospital stay, and preservation of renal function measured by eGFR. Contrary to the renorrhaphy approaches mentioned above, "Off-Clamp, Non-renorrhaphy" technique was more recently introduced in LPN [23]. In this technique, biologic hemostatic agents such as FLOSEAL and TISSEEL are used in the bed of the excised mass as a hemostatic method following renal mass excision without renal artery clamping, and the perirenal fat and Gerota's fascia reapproximation are performed using 3-0 Vicryl continuous sutures without renorrhaphy. Although zero ischemia time was achieved, a high postoperative complication rate (25%), including delayed bleeding requiring blood transfusion, urine leak, and perirenal abscess, may be a concern.
The SLT applied to renorrhaphy in our study was a modification of the existing sliding clip technique, which is at present the most commonly used renorrhaphy method, especially in RALPN [56]. In the case of sliding clip renorrhaphy, suture tension is created by sliding nonabsorbable Weck clips (i.e., Hem-O-Lok clips) along the thread and adding LapraTy clips to prevent the Weck clips from sliding back [5]. These procedures are also applied in our SLT. However, surgical clips used for renorrhaphy may slip or migrate and loosen the compression, causing approximated parenchymal dehiscence or rebleeding [24]. To counter these drawbacks, we designed the suture material with a loop at the end of the thread, and identified the feasibility of SLT through preclinical animal experiments using a porcine model [13]. The potential advantages of SLT renorrhaphy are as follows. First, in our SLT, the application of greater tension would be possible outside the renal parenchyma as well as at the site of the thread passed through the loop, while sliding clip technique provides tension only at the renal parenchyma through which the thread is passed. Consequently, our SLT can apply superior tension without injury, and allow tighter renal parenchymal suturing without dehiscence. In fact, it was confirmed in our previous porcine model experiment that mean distance between renal surfaces after SLT renorrhaphy was significantly narrower than that of the conventional sliding clip technique (1.80 mm vs. 5.28 mm, p<0.001) [13]. Second, in the current study, no postoperative complications have been observed as of the last follow-up for any patient. Although there were 3 cases of intraoperative transfusions, these resulted from causes irrelevant to SLT, including small vein bleeding during hilar dissection and low preoperative hemoglobin level. In contrast, it was reported that there were postoperative perirenal hematoma and anemia as complications related to sliding clip renorrhaphy [6]. Third, our mean WIT was 22.6 minutes, which is comparable to that (22.8 minutes) of sliding clip technique and superior to other studies [61126]. There was no significant decrease in renal function as measured by serum Cr level and eGFR in this study. Furthermore, sliding loop renorrhaphy could be rapidly conducted, as evidenced by the fact that mean RT was 9.0 minutes, accounting for less than half (about 40%) of the acceptable mean WIT. Therefore, SLT in RALPN may be a feasible renorrhaphy method in the light of renal function preservation and time-saving advantages.
The results of our study should be carefully interpreted in the light of several limitations. First, the study design was a case-series with a small volume, and was retrospective in nature, meaning some degree of bias was unavoidable. Second, because the study included patients who underwent surgery performed by multiple surgeons (CWJ and CK), there may be technical variations between surgeons that may have an influence on surgical outcomes. In fact, there were statistically significant differences among surgery-related parameters, including RT, WIT, operative time, and console time. However, our study might be meaningful as an initial preliminary report demonstrating the potential feasibility of SLT in real clinical practice. Further large-scale, prospective, long-term follow-up, and direct comparative studies including other techniques will be required to confirm the clinical applicability of SLT.
In RALPN, SLT was identified as a potentially feasible and safe method for renorrhaphy in terms of tightened renal parenchymal closure, rapid performance, renal function preservation, and low risk of complications in real clinical practice. Further large scale, prospective, longterm follow-up, and direct comparative studies with other techniques are required to confirm the clinical applicability of SLT.
Figures and Tables
Fig. 1
The placement and location of trocars in our four arm approach robot-assisted laparoscopic partial nephrectomy (right side). C, trocar for camera; R1, robotic trocar for 1st arm; R2, robotic trocar for 2nd arm; R3, robotic trocar for 3rd arm; A1, 12-mm trocar for assistant; A2, 5-mm trocar for assistant.
Fig. 2
Brief description of renal reconstruction. (A) The repair of renal parenchymal defect with continuous running suture using 3-0 V-Loc unidirectional barbed suture material. (B) After each suture material is placed through renal capsule, the needle passes through pre-made loop. (C) Weck Hem-O-Lok (Teleflex, Research Triangle, NC, USA) clip is placed along the thread and the clip is pushed toward the renal parenchyma to obtain adequate tension. (D) LapraTy (Ethicon Inc., Cincinnati, OH, USA) clip is added to prevent Weck clips from sliding back and to preserve tension. Scan this QR code to see the accompanying video, or visit www.kjurology.org or http://youtu.be/UCxubKQxQzA.

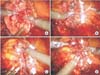
Fig. 3
Suture material designed for sliding loop renorrhaphy. 1-0 Vicryl (approximately 13-cm-long length) with a loop at the end of the thread. A TFE polymer pledget (Ethicon Inc, Somerville, NJ, USA) and a 10-mm Weck Hem-O-Lok (Teleflex, Research Triangle, NC, USA) is placed below the knot.

Table 1
Demographics and perioperative outcomes of patients

References
1. Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009; 182:1271–1279.
2. Touijer K, Jacqmin D, Kavoussi LR, Montorsi F, Patard JJ, Rogers CG, et al. The expanding role of partial nephrectomy: a critical analysis of indications, results, and complications. Eur Urol. 2010; 57:214–222.
3. Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR Jr, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007; 178:41–46.
4. Singh I. Robot-assisted laparoscopic partial nephrectomy: current review of the technique and literature. J Minim Access Surg. 2009; 5:87–92.
5. Bhayani S, Figenshau R. The Washington university renorrhaphy for robotic partial nephrectomy: a detailed description of the technique displayed at the 2008 World Robotic Urologic Symposium. J Robot Surg. 2008; 2:139–140.
6. Benway BM, Wang AJ, Cabello JM, Bhayani SB. Robotic partial nephrectomy with sliding-clip renorrhaphy: technique and outcomes. Eur Urol. 2009; 55:592–599.
7. Benway BM, Cabello JM, Figenshau RS, Bhayani SB. Sliding-clip renorrhaphy provides superior closing tension during robot-assisted partial nephrectomy. J Endourol. 2010; 24:605–608.
8. Sammon J, Petros F, Sukumar S, Bhandari A, Kaul S, Menon M, et al. Barbed suture for renorrhaphy during robot-assisted partial nephrectomy. J Endourol. 2011; 25:529–533.
9. Hillyer S, Spana G, White MA, Autorino R, Laydner H, Khanna R, et al. Novel robotic renorrhaphy technique for hilar tumours: 'V' hilar suture (VHS). BJU Int. 2012; 109:1572–1577.
10. Olweny EO, Park SK, Seideman CA, Best SL, Cadeddu JA. Selfretaining barbed suture for parenchymal repair during laparoscopic partial nephrectomy; initial clinical experience. BJU Int. 2012; 109:906–909.
11. Jeon SH, Jung S, Son HS, Kimm SY, Chung BI. The unidirectional barbed suture for renorrhaphy during laparoscopic partial nephrectomy: Stanford experience. J Laparoendosc Adv Surg Tech A. 2013; 23:521–525.
12. Wahafu W, Ma X, Li HZ, Ding Q, Wang BJ, Shi TP, et al. Evolving renorrhaphy technique for retroperitoneal laparoscopic partial nephrectomy: single-surgeon series. Int J Urol. 2014; 21:865–873.
13. Lee JK, Oh JJ, Lee S, Lee SB, Byun SS, Lee SE, et al. A new sliding-loop technique in renorrhaphy for partial nephrectomy: a feasibility study in a porcine model. Surg Innov. 2015; 07. 12. [Epub]. DOI: 10.1177/1553350615595321.
14. Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001; 166:6–18.
15. McDougall EM, Clayman RV, Chandhoke PS, Kerbl K, Stone AM, Wick MR, et al. Laparoscopic partial nephrectomy in the pig model. J Urol. 1993; 149:1633–1636.
16. Winfield HN, Donovan JF, Godet AS, Clayman RV. Laparoscopic partial nephrectomy: initial case report for benign disease. J Endourol. 1993; 7:521–526.
17. Gettman MT, Blute ML, Chow GK, Neururer R, Bartsch G, Peschel R. Robotic-assisted laparoscopic partial nephrectomy: technique and initial clinical experience with DaVinci robotic system. Urology. 2004; 64:914–918.
18. Beasley KA, Al Omar M, Shaikh A, Bochinski D, Khakhar A, Izawa JI, et al. Laparoscopic versus open partial nephrectomy. Urology. 2004; 64:458–461.
19. Porpiglia F, Volpe A, Billia M, Scarpa RM. Laparoscopic versus open partial nephrectomy: analysis of the current literature. Eur Urol. 2008; 53:732–742.
20. Gettman MT, Blute ML, Peschel R, Bartsch G. Current status of robotics in urologic laparoscopy. Eur Urol. 2003; 43:106–112.
21. Mullins JK, Feng T, Pierorazio PM, Patel HD, Hyams ES, Allaf ME. Comparative analysis of minimally invasive partial nephrectomy techniques in the treatment of localized renal tumors. Urology. 2012; 80:316–321.
22. Zhang X, Shen Z, Zhong S, Zhu Z, Wang X, Xu T. Comparison of peri-operative outcomes of robot-assisted vs laparoscopic partial nephrectomy: a meta-analysis. BJU Int. 2013; 112:1133–1142.
23. Kim TS, Oh JH, Rhew HY. "Off-clamp, non-renorrhaphy" laparoscopic partial nephrectomy with perirenal fat and Gerota's fascia reapproximation: initial experience and perioperative outcomes. J Laparoendosc Adv Surg Tech A. 2014; 24:339–344.
24. Ramanathan R, Leveillee RJ. A review of methods for hemostasis and renorrhaphy after laparoscopic and robot-assisted laparoscopic partial nephrectomy. Curr Urol Rep. 2010; 11:208–220.
25. Khalifeh A, Autorino R, Hillyer SP, Kaouk JH. V-hilar suture renorrhaphy during robotic partial nephrectomy for renal hilar tumors: preliminary outcomes of a novel surgical technique. Urology. 2012; 80:466–471.
26. Orvieto MA, Chien GW, Tolhurst SR, Rapp DE, Steinberg GD, Mikhail AA, et al. Simplifying laparoscopic partial nephrectomy: technical considerations for reproducible outcomes. Urology. 2005; 66:976–980.
SUPPLEMENTARY MATERIAL
An accompanying video can be found in the 'urology in motion' section of the journal homepage (www.kjurology.org). The supplementary data can also be accessed by scanning a QR code located on the Fig. 2 of this article, or be available on YouTube (http://youtu.be/UCxubKQxQzA).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download