Abstract
Purpose
To investigate the difference in rectal complications rate following prostate low dose rate (LDR) brachytherapy based on prostate-rectum distance and prostate longitudinal length among early prostate cancer patients.
Materials and Methods
From March 2008 to February 2013, 245 prostate cancer patients with a Gleason score ≤7 were treated with 125-I LDR brachytherapy. Among them, 178 patients with prostate volume 20-35 mL and a follow-up period ≥6 months were evaluated for radiation proctitis. Magnetic resonance imaging (MRI) was performed for a prebrachytherapy evaluation, and prostate-rectum distance and prostate longitudinal length were measured. The radiation proctitis was confirmed and graded via colonoscopy based on the radiation therapy oncology group (RTOG) toxicity criteria.
Results
Twenty-three patients received a colonoscopy for proctitis evaluation, and 12 were identified as grade 1 on the RTOG scale. Nine patients were diagnosed as grade 2 and 2 patients were grade 3. No patient developed grade 4 proctitis. The rectal-complication group had a mean prostate-rectum distance of 2.51±0.16 mm, while non-rectal-complication control group had 3.32±0.31 mm. The grade 1 proctitis patients had a mean prostate-rectum distance of 2.80±0.15 mm, which was significantly longer than 2.12±0.31 mm of grades 2 and 3 patient groups (p=0.045). All 11 patients of grades 2 and 3 had a prostate longitudinal length of 35.22±2.50 mm, which was longer than group 1, but the difference was not statistically significant (p=0.214).
Prostate cancer is the fifth most common malignancy in Korea and the seventh leading cause of cancer death in men. Prostate brachytherapy is increasingly utilized for localized prostate carcinomas [12]. The advantages of this process are the ease of administration and a lower morbidity compared with a radical prostatectomy or external beam radiation therapy (EBRT), and the reported benefits of prostate brachytherapy compared with a radical prostatectomy and EBRT include lower rates of incontinence and sexual dysfunction [34]. However, prostate brachytherapy can result in radiation proctitis because the rectum is located adjacent to the prostate and is in a fixed position, indicating that the rectum is often exposed to a large dose of radiation in prostate brachytherapy. Although there have been many reports regarding urinary morbidity following brachytherapy, which affects nearly all patients to some degree, there are relatively little data regarding the rectal morbidity [56].
Nonetheless, the rate of radiation proctitis following brachytherapy has been consistently reported to be between 1% and 9%, and rectal bleeding as a late sequela has been reported in approximately 2%-10% of patients, most frequently from 6-18 months after the implantation [67].
Therefore, a cohort of patients with prostate cancer was analyzed to assess the implantation-induced rectal complications and the clinical correlations in this study.
The data summarized in this analysis were collected to clarify the clinical relevance of the magnetic resonance imaging (MRI)-based distance between the prostate capsule and the rectum in two groups of prostate cancer patients, where one group showed endoscopically proven radiation proctitis and the other group did not show any endoscopic abnormalities.
The study approval was obtained from the CHA Bundang Medical Center Institutional Review Board (IRB No. BD 2015-037). From March 2008 to February 2013, a total of 245 men with biopsy-proven prostate cancer and a Gleason score equal to or less than 7 were treated with I-125 brachytherapy. To minimize the size effect of the prostate, 178 of the patients with a prostate volume between 20 and 35 mL and a follow-up period longer than 6 months were selected and evaluated for radiation proctitis. The median follow-up period for overall 178 patients was 37 months. All of the patients underwent an MRI for a prebrachytherapy evaluation, and the prostate-rectum distance and longitudinal length of the prostate were measured using the median-sagittal view of the preimplant magnetic resonance image to analyze the clinical relevance of the corresponding factors and the presence of implantation-induced proctitis. The distance between the prostate and rectum was determined by measuring the shortest distance from the posterior prostate capsule to the anterior wall of the rectum.
One urologist and one radiation oncologist, using a modified peripheral iso-dose plan, performed all of the implantations. For the brachytherapy, a transperineal implantation was undertaken using biplanar ultrasonography with preloaded needles, and an intraoperative cystoscopy was initially performed in all of the patients. A computed tomography (CT) scan was performed after 3-4 weeks to assess the implant quality.
Patients with symptoms of rectal frequency, urgency, tenesmus or bleeding were initially treated with a mesalamine suppository and a soft diet, but if the patients were unresponsive to these therapies, they were referred to a gastroenterologist and evaluated for radiation proctitis via a colonoscopy. The severity of the proctitis was graded using the radiation therapy oncology group (RTOG) scoring criteria.
Next, all of the patients were divided into two groups: one group included the patients with no rectal symptoms or complications, and the other group had colonoscopy-confirmed radiation proctitis. Student t-test was used to estimate the significance of any differences in the prostate-rectum distance and the prostate longitudinal length between the groups. In addition, 2D Pelvis CT images were obtained 30 days after low dose rate (LDR)-brachytherapy for analysis of postimplantation dosimetry and rectal complication. The dosimetric quantifiers included V100 (volume of the prostate receiving 100% of the prescription dose), D90 (radiation dose delivered to 90% of the prostate) and the mean maximum rectal dose (mean maximum radiation delivered to rectum) so that the effect of prostate-rectum distance on rectal doses was studied. The clinical factors that showed a significance in the comparison of the rectal and the nonrectal complication groups were then included in a multivariate analysis using the logistic regression analysis. Analyses were carried out using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). Differences were regarded as statistically significant at p<0.05.
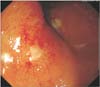
Among the 178 patients who received LDR brachytherapy, 155 patients did not show any rectal symptoms, though the remaining 23 patients showed rectal symptoms. A colonoscopy was performed on all of the patients with rectal complications. Twelve patients (52.2%) were identified as grade 1 according to the RTOG scale because they showed tenesmus, rectal discomfort during defecation and mucinous rectal discharge. Nine patients (39.1%) were diagnosed as grade 2 due to intermittent rectal bleeding, and 2 patients (8.7%) were grade 3 because deep rectal ulcerations were observed in the colonoscopy (Fig. 1). None of the patients developed massive rectal bleeding or grade 4 complications following the LDR brachytherapy, which would require vascular support and emergency colonoscopy with fulguration.
The implant prescription dose was 145 Gy using 71-83 I-125 seeds with a seed activity of 1.413 MBq (0.382 mCi); no supplemental external beam radiation was used. The mean duration for the appearance of the first rectal complication symptom was 8.3±3.9 months after the implantation.
The overall mean prostate-rectum distance, which was measured using the midsagittal view of the pelvic MRI, was 3.21±0.43 mm for the LDR brachytherapy patients. The rectal-complication group had a mean prostate-rectum distance of 2.51±0.16 mm, which was smaller than the mean distance of 3.32±0.31 mm that was observed in the non-rectal-complication control group (Table 1). Within the rectal-complication group, the patients diagnosed as grade 1 had a mean prostate-rectum distance of 2.80±0.15 mm, which was significantly longer than the mean distance of 2.12±0.31 mm that was observed in the grade 2 and 3 patients (p=0.045). The average prostate longitudinal length of overall LDR-brachytherapy patients was 30.42±3.52 mm, and the non-rectal-complication group had an average prostate longitudinal length of 30.08±5.75 mm, which was shorter than 34.12±2.91 mm of the rectal-complication group and the difference was not statistically significant (p=0.102). In the rectal-complication group, all 11 patients diagnosed as grades 2 or 3 of RTOG had an average prostate longitudinal length of 35.22±2.50 mm, which was longer than 32.03±3.64 mm of grade 1 patients, but the difference was not statistically significant (p=0.214). In 30 days postimplantation dosimetry, the mean maximum rectal dose was 161±85 Gy for the non-rectal-complication group and 523±102 Gy for the rectal-complication group (p=0.017). Moreover, the mean maximum rectal dose was substantially increased in the rectal-complication group as RTOG grade increased (p=0.069) (Table 2). V100 and D90 of postimplantation dosimetry analysis revealed no statistically significant difference between the non-rectal-complication group and the rectal-complication group (V100, p=0.190; D90, p=0.410). The multivariate analysis showed the mean maximum rectal dose and the prostate-rectum distance were the significant predictors of the rectal complications (Table 3). Apart from the mean maximum rectal dose and the prostate-rectum distance, other clinical factors such as age of the patients and the prostate longitudinal length were not significant predictors of the rectal complications.
Using the midsagittal view of the MRI image, it was observed that the prostate had different shapes as the prostate-rectum distance changed and the shapes of prostate were divided into three types (Fig. 2). In type 1 individuals, where the prostate-rectum distance was relatively large, the prostate had a triangular shape. In types 2 and 3, the prostate shape changed from an inverted bell to an ovoid shape as the prostate-rectum distance became shorter.
Brachytherapy in early stage prostate cancer has immense advantages compared with a radical prostatectomy, such as fewer urinary complications and less erectile dysfunction. However, the long-term complications of brachytherapy may significantly influence the quality of life due to the prolonged survival after treatment. The incidence of rectal complications is uncommon, which was also observed in this study [89101112131415161718192021]. The majority of the available brachytherapy-related studies have reported incidence rates of rectal ulceration or fistula formation of 1% or less [1011121415].
As many studies have reported a mild degree of rectal toxicity in 15%-39% of postimplantation patients, which resolves spontaneously in most cases [72223], 10 of the 12 patients (83.3%) with grade 1 proctitis showed a spontaneous resolution of the rectal complications in this study. In previous studies, patients with post-implantation rectal complications had a statistically greater length of rectal mucosa and outer rectal surface area when treated at higher doses. Li et al. [24]. reported that the dose to the rectal mucosa could be accurately determined for a "distended" rectum but not for an empty rectum. Moreover, Wallner et al. [17] was able to define the dose to the rectal wall because an obturator was not placed in the rectum, though the rectal mucosa could not be easily identified. Therefore, in this study, the rectal mucosa length was not included as a possible influencing factor of proctitis; instead, only the prostate-rectal distance was measured.
Snyder et al. [25] reported that the incidence of proctitis is directly related to the area of the rectum that receives the minimal prescribed dose (mPD). Moreover, according to Wallner's report, the rectal complications are not related to the size of the prostate, and all of the rectal complications occur between 11 and 22 months after the brachytherapy [17]. However, since the mPD changes according to the variation in the prostate size, this study only included patients with a prostate size of 20 to 35 mL to avoid any selection bias.
Previous reports also found an association between higher rectal doses and complications, and Wallner et al. [17] and Waterman and Dicker [26] reported that the rectal complications are minimal if the highest a rectal dose is equal to or less than 100 cGy. Moreover, Merrick et al. [27] reported that it is coincidental that 10 mm of the anterior rectal mucosa receives 100% of the prescribed dose, regardless of the choice of isotope or treatment approach. This finding implies that the rectal dose of radiation is the most important factor determining postimplantation rectal complications, and the rectal surface area should be measured to determine the rectal dose. Moreover, in 2D CT based 30 days postimplantation dosimetry of this study, the mean maximum rectal dose was higher in the rectal complication group compared with the non-rectal-complication group and it also increased with a statistical significance as RTOG grade progressed within the rectal complication group. Since there was no statistically significant difference between the non-rectal-complication group and the rectal-complication group in the analysis of other dosimetric quantifiers such as V100 and D90, the prostate-rectal distance must be a major factor influencing post-implantation rectal complication. Although, postimplantation dosimetry was applied in this study, measuring the rectal surface using dose-surface histograms is still not an easy process. Therefore, this study focused on more easily measured factors, such as the prostate-rectal distance and the prostate longitudinal length using the midsagittal view of an MRI, but only the prostate-rectal distance was significantly related to rectal complications. Moreover, the multivariate analysis also revealed that the mean maximum rectal dose and prostate-rectum distance were the significant factors of the rectal complications. This relation is likely due to the variations in the prostate shape, as the prostate-rectal distance changes even if the prostate size is similar in each case (Fig. 2). In turn, the results of this study showed that the prostate-rectal distance is clinically related to the prostate shape and that the prostate-rectal distance is a simple and statistically significant clinical factor for postimplant rectal complications.
Moreover, as the prostate shape of the patients in preimplant MRI was close to ovoid shape (type 3), the prostate-rectal distance became shorter, so that these patients might have a greater risk of postimplantation rectal complication and they are probably more suitable for radical prostatectomy. If LDR-brachytherapy was planned for type patients, different implantation techniques, such as transperineal balloon implementation or transperineal hyaluronic acid injection that should be used to minimize the risk of rectal complications [2829]. On the other hand, the patients with triangular shape prostate (type 1) had a relatively longer prostate-rectal distance so that these patients are recommended for LDR-brachytherapy. In the patients with inverted bell shape prostate (type 2), either LDR-brachytherapy or radical prostatectomy could be applied for a surgical treatment of localized prostate cancer.
Because the American Brachytherapy Society recommends undertaking an MRI during the pre-implant planning of transperineal permanent brachytherapy for prostate cancer, it is assumed that the prostate-rectum distance can be measured and used in many institutions [30].
In addition, it appears that the amount of perirectal fat is the major component of the anatomical space between the prostate and rectum. The perirectal fat can act as an anatomic radiation shield and can provide a margin of error if the radioisotope seeds are misplaced posteriorly [18]. The rate of complications determined in this study will be of more valuable when it is compared with the results of patients who underwent different implantation techniques for localized prostate cancer treatment. In addition, multivariate study design including various prostate size and shape, rectal dosimetric analysis, number of implanted seeds and body mass index difference could be undertaken in further analysis and the research is already underway.
This report discusses the differences in the postbrachytherapy rectal complications based on the prostate-rectum distance and the prostate longitudinal length. Despite the limitations of a relatively small sample size and a short follow-up period, this study provides evidence supporting the use of the prostate-rectum distance as a clinical prognosis factor for postimplantation rectal complications. For prostate LDR-brachytherapy, a preimplantation MRI is very useful for selecting patients, and those with shorter prostate-rectum distances should receive modified implantation techniques or a radical prostatectomy. For different types of prostate and rectum shapes, type I group is recommended for LDR-brachythrerapy, while type 3 group is more suitable for radical prostatectomy or LDR-brachytherapy with modified implantation technique to avoid or minimize post implantation rectal complications.
Figures and Tables
 | Fig. 2Midsaggital view of pelvic magnetic resonance imaging with types of prostate and rectum shape. (A) Type 1, triangular shape with relatively thick prostate-rectum distance; (B) type 2, inverted bell shape with relatively thin prostate-rectum distance; (C) type 3, ovoid shape with direct contact prostate-rectum (minimal distance). P, prostate; R, rectum; RP, right posterior; RPH, relative peak height; LPF, low pass filter. |
Table 1
Characteristics and dosimetric quantifiers of LDR-brachytherapy patients, stratified by presence of rectal symptoms total patient number (n=178)

Table 2
Detailed characteristics of patients with rectal symptoms patient characteristics (n=23)

References
1. Ragde H, Elgamal AA, Snow PB, Brandt J, Bartolucci AA, Nadir BS, et al. Ten-year disease free survival after transperineal sonography-guided iodine-125 brachytherapy with or without 45-gray external beam irradiation in the treatment of patients with clinically localized, low to high Gleason grade prostate carcinoma. Cancer. 1998; 83:989–1001.
2. Blasko JC, Grimm PD, Sylvester JE, Badiozamani KR, Hoak D, Cavanagh W. Palladium-103 brachytherapy for prostate carcinoma. Int J Radiat Oncol Biol Phys. 2000; 46:839–850.
3. Frank SJ, Pisters LL, Davis J, Lee AK, Bassett R, Kuban DA. An assessment of quality of life following radical prostatectomy, high dose external beam radiation therapy and brachytherapy iodine implantation as monotherapies for localized prostate cancer. J Urol. 2007; 177:2151–2156.
4. Wei JT, Dunn RL, Sandler HM, McLaughlin PW, Montie JE, Litwin MS, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002; 20:557–566.
5. Kleinberg L, Wallner K, Roy J, Zelefsky M, Arterbery VE, Fuks Z, et al. Treatment-related symptoms during the first year following transperineal 125I prostate implantation. Int J Radiat Oncol Biol Phys. 1994; 28:985–990.
6. Gelblum DY, Potters L. Rectal complications associated with transperineal interstitial brachytherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2000; 48:119–124.
7. Wallner K, Blasko JC, Dattoli M. Prostate brachytherapy made complicated. 2nd ed. Seattle: Smart Medicine Press;2001.
8. Wallner K, Roy J, Zelefsky M, Fuks Z, Harrison L. Short-term freedom from disease progression after I-125 prostate implantation. Int J Radiat Oncol Biol Phys. 1994; 30:405–409.
9. Wallner K, Roy J, Harrison L. Tumor control and morbidity following transperineal iodine 125 implantation for stage T1/T2 prostatic carcinoma. J Clin Oncol. 1996; 14:449–453.
10. Blasko JC, Ragde H, Cavanagh W, Sylvaster J, Grimm PD. Long term outcomes of external beam irradiation and I-125/Pd-103 brachytherapy boost for prostate cancer. Int J Radiat Oncol Biol Phys. 1996; 36:198.
11. Critz FA, Levinson K, Williams WH, Holladay D, Holladay C, Griffin V. Prostate-specific antigen nadir of 0.5 ng/mL or less defines disease freedom for surgically staged men irradiated for prostate cancer. Urology. 1997; 49:668–672.
12. Critz FA, Levinson AK, Williams WH, Holladay DA. Prostatespecific antigen nadir: the optimum level after irradiation for prostate cancer. J Clin Oncol. 1996; 14:2893–2900.
13. Albert P, Zeitlin S, Raboy A, Lederman G. High dose combination radiotherapy in the treatment of carcinoma of the prostate - 410 consecutive patients. J Urol. 1997; 157:290.
14. Beyer DC, Priestley JB Jr. Biochemical disease-free survival following 125I prostate implantation. Int J Radiat Oncol Biol Phys. 1997; 37:559–563.
15. Stone NN, Stock RG. Brachytherapy for prostate cancer: realtime three-dimensional interactive seed implantation. Tech Urol. 1995; 1:72–80.
16. Stock RG, Stone NN, DeWyngaert JK, Lavagnini P, Unger PD. Prostate specific antigen findings and biopsy results following interactive ultrasound guided transperineal brachytherapy for early stage prostate carcinoma. Cancer. 1996; 77:2386–2392.
17. Wallner K, Roy J, Harrison L. Dosimetry guidelines to minimize urethral and rectal morbidity following transperineal I-125 prostate brachytherapy. Int J Radiat Oncol Biol Phys. 1995; 32:465–471.
18. Kaye KW, Olson DJ, Payne JT. Detailed preliminary analysis of 125iodine implantation for localized prostate cancer using percutaneous approach. J Urol. 1995; 153(3 Pt 2):1020–1025.
19. Phan J, Swanson DA, Levy LB, Kudchadker RJ, Bruno TL, Frank SJ. Late rectal complications after prostate brachytherapy for localized prostate cancer: incidence and management. Cancer. 2009; 115:1827–1839.
20. Benoit RM, Naslund MJ, Cohen JK. Complications after prostate brachytherapy in the Medicare population. Urology. 2000; 55:91–96.
21. Dattoli M, Wallner K, Sorace R, Koval J, Cash J, Acosta R, et al. 103Pd brachytherapy and external beam irradiation for clinically localized, high-risk prostatic carcinoma. Int J Radiat Oncol Biol Phys. 1996; 35:875–879.
22. Lee WR, McQuellon RP, Case LD, deGuzman AF, McCullough DL. Early quality of life assessment in men treated with permanent source interstitial brachytherapy for clinically localized prostate cancer. J Urol. 1999; 162:403–406.
23. Brandeis JM, Litwin MS, Burnison CM, Reiter RE. Quality of life outcomes after brachytherapy for early stage prostate cancer. J Urol. 2000; 163:851–857.
24. Li S, Boyer A, Lu Y, Chen GT. Analysis of the dose-surface histogram and dose-wall histogram for the rectum and bladder. Med Phys. 1997; 24:1107–1116.
25. Snyder KM, Stock RG, Hong SM, Lo YC, Stone NN. Defining the risk of developing grade 2 proctitis following 125I prostate brachytherapy using a rectal dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2001; 50:335–341.
26. Waterman FM, Dicker AP. Probability of late rectal morbidity in 125I prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2003; 55:342–353.
27. Merrick GS, Butler WM, Dorsey AT, Lief JH, Walbert HL, Blatt HJ. Rectal dosimetric analysis following prostate brachytherapy. Int J Radiat Oncol Biol Phys. 1999; 43:1021–1027.
28. Kouloulias V, Kalogeropoulos T, Platoni K, Georgakopoulos J, Matsopoulos G, Chaldeopoulos D, et al. Feasibility and radiation induced toxicity regarding the first application of transperineal implementation of biocompatible balloon for high dose radiotherapy in patients with prostate carcinoma. Radiat Oncol. 2013; 8:82.
29. Prada PJ, Fernandez J, Martinez AA, de la Rua A, Gonzalez JM, Fernandez JM, et al. Transperineal injection of hyaluronic acid in anterior perirectal fat to decrease rectal toxicity from radiation delivered with intensity modulated brachytherapy or EBRT for prostate cancer patients. Int J Radiat Oncol Biol Phys. 2007; 69:95–102.
30. Davis BJ, Horwitz EM, Lee WR, Crook JM, Stock RG, Merrick GS, et al. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy. 2012; 11:6–19.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download