Abstract
Purpose
To determine whether statin use delays the development of castration-resistant prostate cancer (CRPC) in patients with metastatic prostate cancer treated with androgen deprivation therapy (ADT).
Materials and Methods
A total of 171 patients with metastatic prostate cancer at the time of diagnosis who were treated with ADT between January 1997 and December 2013 were retrospectively analyzed. The patients were classified into two groups: the nonstatin use group (A group) and the statin use group (B group). Multivariate analysis was performed on statin use and other factors considered likely to have an effect on the time to progression to CRPC.
Results
The mean patient age was 67.1±9.1 years, and the mean follow-up period was 52 months. The mean initial prostate-specific antigen (PSA) level was 537 ng/mL. Of the 171 patients, 125 (73%) were in group A and 46 (27%) were in group B. The time to progression to CRPC was 22.7 months in group A and 30.5 months in group B, and this difference was significant (p=0.032). Blood cholesterol and initial PSA levels did not differ significantly according to the time to progression to CRPC (p=0.288, p=0.198). Multivariate analysis using the Cox regression method showed that not having diabetes (p=0.037) and using a statin (p=0.045) significantly increased the odds ratio of a longer progression to CRPC.
Prostate cancer is the most commonly diagnosed cancer in men in the United States and the second most commonly diagnosed malignancy worldwide [1]. Although many prostate cancer patients have localized disease at the time of diagnosis, some present with evidence of metastasis. In the latter cases, androgen deprivation therapy (ADT) is the first-line treatment. Although this therapy is very effective initially, the disease eventually progresses in all patients and becomes resistant to treatment, which is also known as castration-resistant prostate cancer (CRPC). CRPC has a poor prognosis and a high mortality rate.
3-Hydroxy-3-methyl-glutaryl-CoA reductase inhibitors, commonly known as statins, are highly effective in lowering cholesterol levels and reducing the risk of cardiovascular disease. However, statins can also modify the cholesterol levels needed for signal transduction and have shown an effect on prostate cancer cells [2]. Statins are thought to modulate androgen receptor expression and activity, which may reduce the proliferation of prostate cancer cells and induce apoptosis [34]. Statins may also reduce the levels of prostate-specific antigen (PSA) released by prostate cancer cells. A number of epidemiological studies have shown a relationship between statin use and lower cancer risk and mortality, including in prostate cancer [56789]. In many studies, statin use has shown an antitumor effect in prostate cancer, decreasing the risk of recurrence and prostate cancer mortality [10]. In patients who have undergone radical prostatectomy, statins also reduce the risk of prostate cancer recurrence [11]. In addition, statins are associated with reduced mortality rates in prostate cancer patients who have been treated with radiation therapy [12].
The aim of our current study was to determine whether statins have an effect on prostate cancer patients who cannot be treated with standard definitive therapies owing to the extent of disease. We investigated whether statin use delays the development of CRPC in metastatic prostate cancer patients who had been treated with ADT.
This study was performed with the approval and oversight of the Institutional Review Board (IRB) of Asan Medical Center (IRB No. 2015-0582). A database of prostate cancer patients who already had metastasis and were treated with ADT between January 1997 and December 2013 at Asan Medical Center was retrospectively analyzed. Because of the retrospective nature of this analysis, the requirement for informed consent was waived by the IRB. A total of 196 patients who had metastatic prostate adenocarcinoma at the time of diagnosis and had been treated with ADT and eventually progressed to CRPC were selected for analysis. The patient characteristics we assessed included age at diagnosis, diabetes mellitus, hypertension, body mass index (BMI), Gleason score for a prostate biopsy, and initial PSA level before ADT. Information on statin medication was collected retrospectively from the patients' medical records. Statin users included all patients who used a statin before and after diagnosis. The primary study end point was the occurrence of CRPC. In addition, cancer-specific survival was assessed.
Clinicopathological characteristics were compared between patients exposed or not to statins by use of the chi-square test and Student t-test. Cox proportional hazards models were used to estimate hazard ratios (HRs) with a 95% confidence interval (CI) for CRPC occurrence to determine the effect of statin use on the time to CRPC onset. The HR of prostate cancer mortality was also estimated. Data were analyzed by using the IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). Significance was defined as p≤0.05.
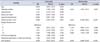
The clinicopathologic characteristics of all the prostate cancer subjects according to statin use are summarized in Table 1. The patients' mean age at diagnosis was 67.1 years (standard deviation, 9.1 years), with a mean follow-up time of 52 months. Statins were used in 46 patients (27.2%). The mean time to CRPC was 25 months after diagnosis. Of the 171 patients evaluated, 132 died of prostate cancer, with a mean survival time after diagnosis of 48.6 months.
Differences in patient characteristics between statin users and nonusers are listed in Table 2. There were no statistically significant differences in the prevalence of comorbidities such as diabetes mellitus or hypertension between the groups. Among patients with diabetes mellitus, 15 patients (48%) had metformin in their medication: 5 patients (10%) in the statin group and 10 patients (8%) in the non statin group. Cross-tabulation analysis showed no statistical difference in the use of metformin between the two groups (p=0.879). Among patients with hypertension, an angiotensin converting enzyme (ACE) inhibitor was included in three patients and a beta-blocker was included in eight patients; one patient used both an ACE inhibitor and a beta-blocker. Cross-tabulation was also done and showed no statistical difference between the two groups for the above medications (p=0.393). Furthermore, there were no significant differences between the study groups in terms of the Gleason score for prostate cancer or the PSA level before treatment (p=0.547, p=0.681). As expected, statin users had a significantly higher BMI (p=0.047). Also, there was a significant difference in the time to development of CRPC between statin users and nonusers with a median time of 30.5 and 22.7 months, respectively (p=0.016).
Treatment after occurrence of CRPC was also analyzed. A total of 115 patients (67%) used docetaxel as chemotherapy after CRPC. A total of 23 patients (13%) did not receive chemotherapy and 33 patients had other treatments such as mitoxantrone, enzalutamide, and cyclophosphamide vincristine dexamethasone combination therapy. Docetaxel was used in 69% of the patients in the statin group and in 67% of the patients in the nonstatin use group. Cross-tabulation analysis showed no significant difference in treatment method after CRPC between the two groups (p=0.701).
The results of our primary analysis using univariate and multivariate HR models with Cox regression analysis are listed in Tables 3, 4. A lower Gleason score was associated with longer time to CRPC onset. In addition, the presence of diabetes mellitus was associated with ADT duration before CRPC (HR, 1.573; 95% CI, 1.02-2.43; p=0.041). Using statins was associated with a longer ADT duration (HR, 0.61; 95% CI, 0.41-0.91; p=0.015), with a difference in the CRPC-free survival curve as shown in Fig. 1 (p=0.044). Among the 171 study patients, 132 died of prostate cancer. Cancer-specific survival was analyzed by using Cox regression analysis. Similar to ADT duration, the Gleason score, diabetes mellitus, and statin use were associated with cancer-specific survival. A higher Gleason score was associated with increased risk of cancer-specific mortality (p=0.002). Diabetes mellitus was also associated with increased risk (HR, 1.641; 95% CI, 1.03-2.61; p=0.037), and statin users showed a lower risk of cancer-specific mortality than did nonusers (HR, 0.405; 95% CI, 0.25-0.66; p<0.001) and also showed a significant difference in the survival curve as shown in Fig. 2 (p=0.001). In addition, a longer duration of ADT use was associated with a lower cancer-specific mortality rate (HR, 0.909; 95% CI, 0.89-0.93; p<0.001).
The antitumor effects of statins in prostate cancer patients have been reported in many previous studies [3456]. In our current study, we evaluated the association between statin use and prognosis of metastatic prostate cancer because statins are known to reduce the risk of advanced prostate cancer. Previous studies have reported that the effects of statins on prostate cancer are linked to several pathways related to cancer development and growth [3]. The results of our current study of metastatic prostate cancer patients indicate that the use of statins is associated with delay in the development of CRPC, even though all patients eventually developed CRPC. One explanation for this result may be that statin users may have more favorable prostate cancer characteristics and better cancer outcomes [131415]. In our present analysis, no significant differences in cancer characteristics were seen between statin users and nonusers. The possibility of healthy-user bias is another important factor that should be considered. Statin users may be more focused on their health and thus may have better lifestyle habits, which could have an effect on prostate cancer survival. However, blood cholesterol levels in patients in the statin user group were high. Also, we noted a reduced HR of 59.5% associated with statin use, which suggests that factors other than lifestyle habits play a role in the effects of statins. Although other confounding factors may have been present in our study cohort, statin users had a higher BMI and higher cholesterol levels, which may be associated with poorer survival and may weaken the effects of the statins. Nevertheless, our current results show that statin use may decrease prostate cancer mortality and delay the development of CRPC.
Statins are also known to lower serum PSA levels, which may influence the development and metastasis of prostate cancer. However, in our current study, we saw no significant difference in PSA levels between the groups: 515 ng/mL and 559 ng/mL in the nonstatin user group and the statin user group, respectively. The discrepancy among studies in this regard may be due to differences in the patient populations. Our present patients all had metastatic prostate cancer at the time of diagnosis, and therefore the burden of prostate cancer may have been higher in this group than in previously reported cohorts. This in turn may have diluted the PSA-lowering effect of statins.
Statins inhibit 3-hydroxy-3-methylglutaryl (HMG) coenzyme A reductase and limit cholesterol biosynthesis by affecting the pathway that converts HMG coenzyme A to mevalonate, eventually reducing the level of mevalonate. The lower level of cholesterol in the blood may limit cholesterol use in cells and modulate the signal pathway associated with cancer apoptosis [16], such as Akt signaling in prostate cancer cells [317181920]. Reduced mevalonate levels lead to deprivation of isoprenoids and thus limit the activation of proteins such as Ras and Rho in the cell membrane, eventually decreasing their growth promotion and survival activities [2122]. According to this hypothesis, statins may have an effect in patients without hypercholesterolemia. In our present study, however, we did not assess any biochemical markers that could be used to support this mechanism. The effect of statins on prostate cancer patients needs further research in prospective studies.
The duration of ADT was also found to be associated with prostate cancer survival, with a longer duration of ADT associated with a lower risk of cancer-specific mortality. A linear correlation was observed between the duration of ADT and the survival time after the initial diagnosis of prostate cancer, as shown by simple correlation analysis (Fig. 3). This may be because patients who received ADT for a longer duration may have had better pathologic features. However, the duration of ADT also showed statistically significant associations with factors that influenced cancer-specific survival in our multivariate analysis.
Diabetes was found to be a statistically significant risk factor for both CRPC and cancer-specific survival. Diabetic patients usually have a higher level of morbidity than do nondiabetic patients, and this may also have an effect on survival; however, the difference in the duration of ADT owing to diabetic status is likely to be attributable to more than just lifestyle differences. Several studies have shown that diabetes is a risk factor for cancer, including prostate cancer, while other studies have reported that diabetes is a protective factor for prostate cancer [2324252627]. The use of glucose-lowering agents such as metformin may also affect prostate cancer development and prognosis [28]. The relationship between diabetes and prostate cancer is not yet clear, and further studies on this subject are currently underway
Although all of our study patients presented with bone metastasis, some patients also had visceral metastasis at the time of diagnosis. The patients who presented with visceral metastasis were expected to have a poorer prognosis and shorter time to CRPC onset, likely as a result of a higher tumor burden than in patients who presented with only a bone metastasis. Surprisingly, however, we found no statistically significant difference in the risk of CRPC and cancer-specific survival in patients with an additional visceral metastasis. Initial PSA levels before treatment also did not significantly differ between patients with only bone metastasis and patients with a visceral metastasis also. This may indicate that a combination of visceral and bone metastases does not correlate with a higher tumor burden and a poorer prognosis.
There were some limitations of note to our study, mainly due to the retrospective nature of the analyses. For example, there may have been gaps in our data, including comorbidities and statin use, owing to an incomplete reporting of medical histories. Records of any existing medications were obtained by querying the patients at the time of admission. The duration of medication use before admission was calculated by using the date provided in the medical records, but the degree of patient compliance with those medication regimens is unknown, which may have biased our calculations. Also, when this study was conducted, only 19 patients (30%) in the statin user group were alive to respond to phone calls about duration of statin use. Therefore, data on total statin duration and the accumulative dose of statins were not appropriately obtained or analyzed. There may also have been data gaps for statin use if any patients failed to report their use. However, such a bias would result in statin users being included in the nonstatin user group, which would weaken the measured effects of statins on prostate cancer.
The use of statins is associated with a delayed development of CRPC and also with lower prostate cancer-specific survival. Although statin use may also be associated with a decreased risk of prostate cancer mortality, additional prospective evaluations of the impact of statins are needed.
Figures and Tables
 | Fig. 1CRPC-free survival outcomes between statin users and nonusers. CRPC, castration-resistant prostate cancer. |
Table 1
Clinicopathological characteristics of the study patients (n=171)

Table 2
Clinicopathological characteristics of the study patients according to statin use

Table 3
Univariate and multivariate analysis of factors affecting ADT duration prior to CRPC onset

Table 4
Univariate and multivariate analysis of factors affecting cancer specific survival
ACKNOWLEDGMENTS
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea.
References
1. Stewart BW, Wild CP. World cancer report 2014. Geneva: World Health Organization;2014.
2. Hamilton RJ, Goldberg KC, Platz EA, Freedland SJ. The influence of statin medications on prostate-specific antigen levels. J Natl Cancer Inst. 2008; 100:1511–1518.
3. Yang L, Egger M, Plattner R, Klocker H, Eder IE. Lovastatin causes diminished PSA secretion by inhibiting AR expression and function in LNCaP prostate cancer cells. Urology. 2011; 77:1508.e1–1508.e7.
4. Yokomizo A, Shiota M, Kashiwagi E, Kuroiwa K, Tatsugami K, Inokuchi J, et al. Statins reduce the androgen sensitivity and cell proliferation by decreasing the androgen receptor protein in prostate cancer cells. Prostate. 2011; 71:298–304.
5. Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007; 16:2226–2232.
6. Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol. 2004; 22:2388–2394.
7. Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006; 98:1819–1825.
8. Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, Rennert HS, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005; 352:2184–2192.
9. Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012; 367:1792–1802.
10. Blais L, Desgagne A, LeLorier J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med. 2000; 160:2363–2368.
11. Hamilton RJ, Banez LL, Aronson WJ, Terris MK, Platz EA, Kane CJ, et al. Statin medication use and the risk of biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer. 2010; 116:3389–3398.
12. Oh DS, Koontz B, Freedland SJ, Gerber L, Patel P, Lewis S, et al. Statin use is associated with decreased prostate cancer recurrence in men treated with brachytherapy. World J Urol. 2015; 33:93–97.
13. Breau RH, Karnes RJ, Jacobson DJ, McGree ME, Jacobsen SJ, Nehra A, et al. The association between statin use and the diagnosis of prostate cancer in a population based cohort. J Urol. 2010; 184:494–499.
14. Loeb S, Kan D, Helfand BT, Nadler RB, Catalona WJ. Is statin use associated with prostate cancer aggressiveness? BJU Int. 2010; 105:1222–1225.
15. Farwell WR, D'Avolio LW, Scranton RE, Lawler EV, Gaziano JM. Statins and prostate cancer diagnosis and grade in a veterans population. J Natl Cancer Inst. 2011; 103:885–892.
16. Pelton K, Freeman MR, Solomon KR. Cholesterol and prostate cancer. Curr Opin Pharmacol. 2012; 12:751–759.
17. Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005; 115:959–968.
18. Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002; 62:2227–2231.
19. Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem. 2004; 91:54–69.
20. Twiddy AL, Cox ME, Wasan KM. Knockdown of scavenger receptor class B type I reduces prostate specific antigen secretion and viability of prostate cancer cells. Prostate. 2012; 72:955–965.
21. Graaf MR, Richel DJ, van Noorden CJ, Guchelaar HJ. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev. 2004; 30:609–641.
22. Mo H, Elson CE. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp Biol Med (Maywood). 2004; 229:567–585.
23. Anwar MA, Kheir WA, Eid S, Fares J, Liu X, Eid AH, et al. Colorectal and prostate cancer risk in diabetes: metformin, an actor behind the scene. J Cancer. 2014; 5:736–744.
24. Deng L, Gui Z, Zhao L, Wang J, Shen L. Diabetes mellitus and the incidence of colorectal cancer: an updated systematic review and meta-analysis. Dig Dis Sci. 2012; 57:1576–1585.
25. Kasper JS, Liu Y, Giovannucci E. Diabetes mellitus and risk of prostate cancer in the health professionals follow-up study. Int J Cancer. 2009; 124:1398–1403.
26. Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care. 2015; 38:264–270.
27. Waters KM, Henderson BE, Stram DO, Wan P, Kolonel LN, Haiman CA. Association of diabetes with prostate cancer risk in the multiethnic cohort. Am J Epidemiol. 2009; 169:937–945.
28. Hwang IC, Park SM, Shin D, Ahn HY, Rieken M, Shariat SF. Metformin association with lower prostate cancer recurrence in type 2 diabetes: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2015; 16:595–600.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download