Abstract
Purpose
Few data are available concerning the clinical outcome of abiraterone acetate treatment in patients with metastatic castration-resistant prostate cancer (mCRPC) in terms of the duration of androgen deprivation therapy (ADT) before diagnosis of CRPC. We investigated the clinical efficacy of abiraterone acetate according to the duration of ADT.
Materials and Methods
We reviewed the medical records of 20 patients with mCRPC who received abiraterone acetate after failure of docetaxel chemotherapy from May 2012 to March 2014 at Seoul National University Bundang Hospital. Clinical factors including prostate-specific antigen (PSA) nadir level, time to PSA nadir, PSA doubling time, PSA response, and modes of progression (PSA, radiologic, clinical) were analyzed. Disease progression was classified according to the Prostate Cancer Working Group 2 criteria.
Results
The mean age and PSA value of the entire cohort were 76.0±7.2 years and 158.8±237.9 ng/mL, respectively. The median follow-up duration was 13.4±6.7 months. There were no statistically significant differences in clinical characteristics between patients who received abiraterone acetate with ADT duration<35 months and those who received abiraterone acetate with ADT duration≥35 months. There were also no significant differences in terms of PSA progression-free survival, radiologic progression-free survival, and clinical progression-free survival between patients with ADT duration<35 months and those with ADT duration ≥35 months.
Conclusions
Although this was a retrospective study with a small sample size, we did not observe any statistically significant differences in the clinical response to abiraterone acetate between mCRPC patients with long ADT duration and those with short ADT duration in terms of disease progression-free survival.
The novel therapeutic agents for metastatic castration-resistant prostate cancer (mCRPC) have rapidly expanded in recent years. Accordingly, the treatment options for mCRPC have recently expanded [12]. Abiraterone acetate and enzalutamide were introduced as second-line hormone therapies for patients with mCRPC after treatment failure of docetaxel chemotherapy. Other second-line treatments are sipuleucel-T (IMPACT [Identification of Men with a genetic predisposition to ProstAte Cancer: Targeted screening in men at a higher genetic risk and controls] study) [3], Alpharadin (ALSYMPCA [Alpharadin in Symptomatic Prostate Cancer Patients] study) [4], and cabazitaxel (TROPIC [Phase III trial of cabazitaxel for the treatment of metastatic castration-resistant prostate cancer] study) [5].
Abiraterone acetate (abiraterone) is one of the novel agents for treating mCRPC. Abiraterone is an orally available inhibitor of CYP17, which is an enzyme centrally involved in the extragonadal synthesis of androgens and estrogens [6]. Moreover, abiraterone is an irreversible and potent inhibitor of cytochrome P450 17 (CYP17). Abiraterone (Zytiga, Janssen, CA, USA) was approved by the U.S. Food and Drug Administration in April 2011.
In the COU-AA-301 trial, abiraterone was shown to improve overall survival (OS) and quality of life in patients with advanced prostate cancer in the last 2 years of treatment [7]. Furthermore, in the COU-AA-302 trial, abiraterone was shown to improve radiographic progression-free survival in docetaxel-naïve patients [8].
The activity of abiraterone according to Eastern Cooperative Oncology Group (ECOG) performance status (PS) is not well described. In clinical practice, abiraterone is usually used in ECOG PS≥2 patients [9]. In real clinical practice, however, clinical outcomes in Asian populations are insufficient owing to a lack of clinical data. In previous studies, a relatively small number of ECOG PS≥2 patients were included. Abiraterone acetate is expensive and the patients who can be administered abiraterone are limited. Also, few data are available regarding the clinical effects of abiraterone in patients with mCRPC according to duration of androgen deprivation therapy (ADT). In the present study, therefore, we analyzed the efficacy of abiraterone in terms of ADT duration and present a representative case for an ECOG PS 2 patient.
With Institutional Review Board approval, we retrospectively analyzed the medical records of patients who took abiraterone from May 2012 to March 2014 at Seoul National University Bundang Hospital (IRB approval number B-1408/264-107). For each patient, the following clinicopathologic features were considered: age, body mass index (BMI), prostate-specific antigen (PSA), number of metastatic sites, biopsy Gleason score, previous radiotherapy history, previous radical prostatectomy history, previous ADT history, and previous chemotherapy history. mCRPC patients who failed docetaxel-based chemotherapy were treated with abiraterone (1,000 mg, once a day) and prednisolone (5 mg, twice a day).
In the assessment of clinical outcomes according to ADT duration (group A, ADT duration<35 months; group B, ADT duration≥35 months), the following variables were recorded: nadir PSA, PSA decline≥50%, duration of PSA decline≥50%, side effects, PSA response, duration of treatment, PSA doubling time, PSA progression, radiologic progression, and clinical progression. We categorized patients according to mean ADT duration (34.5 months). Because it did not relatively differ from our median duration (29 months), the mean duration, which was approximately 3 years, was selected for the analysis. The duration of previous ADT was defined as time from start of first-line hormonal therapy to start date of first subsequent anticancer treatment. Side effects were classified according to the Common Terminology Criteria for Adverse Events. Side effects were defined as a grade of 3 or higher according to the criteria. PSA response was defined as a decline in PSA level of 30% or more. PSA doubling time was defined as the time it took for the PSA value to double from the PSA nadir level. PSA progression, radiologic progression, and clinical progression were defined according to the Prostate Cancer Working Group 2. In addition, we analyzed clinical outcomes according to ECOG PS.
The baseline characteristics of the patients were analyzed by using Mann-Whitney U-test and Fisher exact-test. Fisher exact-test also was used to analyze the stratified clinical response to abiraterone according to ADT duration. PSA progression, radiologic progression, and clinical progression according to stratified ADT duration were compared by use of Kaplan-Meier analysis with a log-rank test. The impact of various clinicopathological factors on progression-free survival was examined by using univariate and multivariate Cox proportional hazards regression models, and hazards ratios and 95% confidence intervals were computed. All data analyses were performed by using the PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). A p-value of <0.05 was considered statistically significant.
The baseline characteristics of the patients stratified by mean ADT duration are presented in Table 1. Group A consisted of 11 patients and group B included 9 patients. The mean patient age for this cohort was 75.95±7.2 years, the mean BMI was 24.15±3.1 kg/m2, and the mean PSA was 158.82±237.9 ng/mL. The number of metastases in each group was almost one site. A similar proportion of patients in groups A and B were undergoing radical prostatectomy or radiation therapy. Additional detailed patient characteristics are presented in Table 1. The median follow-up duration was 13.4±6.7 months.
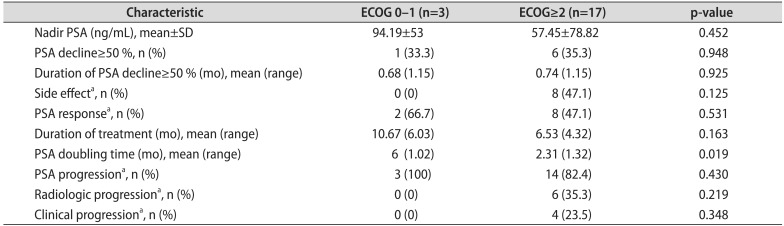
Treatment outcomes with abiraterone in groups A and B are presented in Table 2. The mean nadir PSA was 80.3 ng/mL in group A; the mean nadir PSA was 41.7 ng/mL in group B. Significant PSA decline (≥50%) was observed in two patients (18.2%) in group A and in five patients (55.6%) in group B, respectively. Side effects (grade 3 or greater) were observed in eight patients (47.1%) in the entire cohort (group A: four patients [36.4%] vs. group B: four patients [44.4%]). Additional detailed treatment outcomes are presented in Table 2. There were no statistically significant differences in outcomes with abiraterone acetate in patients stratified by ECOG PS 1-0 or ECOG PS≥ 2 (Table 3). In the Kaplan-Meier analysis, the PSA progression of group A was not significantly different from that of group B (log rank test; p=0.322). As for radiologic progression, there was no significant difference between groups A and B (log rank test; p=0.717). As for clinical progression, there was also no significant difference between groups A and B (log rank test; p=0.298). In the multivariable analysis, there was no statistically significant difference in outcomes with abiraterone acetate between patients with ADT duration<35 months and those with ADT duration≥35 months.
We treated a man who was born in 1936 and had metastatic prostate cancer since 2010. His prostate cancer was diagnosed in 1999. He underwent radical prostatectomy in 1999 and was reported margin positive in superior and inferior with pathologic stage T2cN0M0, Gleason score 8 (4+4), and no lymph node invasion. In 2004, his PSA level during follow-up was 10.1 ng/mL. At that time, treatment with maximal androgen blockade with antiandrogen and luteinizing hormone-releasing hormone (LHRH) agonist was tried. Side effects of hormone therapy appeared as gynecomastia and an elevated blood glucose level. We changed the regimen to antiandrogen monotherapy and stopped the LHRH agonist. Since 2005, with his PSA decreasing to 0.1 ng/mL, we adapted intermittent hormonal treatment. In 2010, we found a metastatic lung lesion incidentally. We performed lung biopsy and the biopsy result was reported as metastatic adenocarcinoma from prostate cancer. A bone scan showed multiple bone metastases at that time. We started an LHRH antagonist (Firmagon, Ferring, Switzerland), but the treatment was not effective. We started docetaxel chemotherapy and performed 8 cycles. Despite docetaxel chemotherapy, his PSA gradually increased. A follow-up bone scan showed aggravation of multiple bone metastases. In January 2013, we started abiraterone acetate treatment. He had a dramatic response to abiraterone treatment and his PSA level declined from 44.92 to 0.006 ng/mL. Follow-up bone scan and chest computed tomography showed dramatically reduced lesions compared with previous lesions. However, we stopped abiraterone treatment temporarily because of fluid retention. After the abiraterone treatment was stopped, the patient's PSA level increased to 3.474 ng/mL. We restarted abiraterone and his PSA level declined significantly again. This patient experienced more than 24 months of progression-free survival.
A paucity of data exists in the literature regarding the clinical effects of abiraterone acetate in patients with mCRPC according to ADT duration, although the duration of ADT was previously reported as a potential prognostic factor in men with mCRPC receiving second-line chemotherapy [10]. To our knowledge, our study is the first to exclusively focus on the clinical effects of abiraterone acetate in Asian patients with mCRPC according to ADT duration. Duration of hormone therapy might reflect the biological prognosis of underlying prostate cancer, specifically, the response to antiandrogen therapies. In the current study, we found that response to abiraterone acetate may be similar between patients with ADT duration<35 months and those with ADT duration≥35 months. We found no significant differences in terms of the proportion of PSA progression, radiologic progression, and clinical progression between the two groups. In contrast with our data, Chi et al. [11] reported that a longer duration of ADT was associated with favorable outcomes in mCRPC patients treated with abiraterone.
The baseline serum androgen level in mCRPC patients treated with abiraterone acetate was reported as a prognostic factor for OS in the post-docetaxel setting [12]. Although mCRPC patients with low baseline serum androgen levels also benefited from abiraterone acetate compared with a placebo group in the COU-AA-301 trial, patients with high baseline androgen had better OS rates than did those with low baseline serum androgen. These data might suggest that prostate cancer in a very low testosterone environment represents more aggressive biological features associated with decreased survival compared with prostate cancer that remains more dependent on stimulation by circulating androgen. However, we could not assess serum androgen levels before treatment with abiraterone acetate.
Previously, Azad et al. [13] performed a study to determine the relative importance of ECOG PS. They analyzed 519 patients with an ECOG PS of 0-1 (n=318, 61%) or an ECOG PS of 2 (n=201, 39%). They reported that patients with a low ECOG PS were significantly more likely to achieve significant PSA decline from baseline (PSA decline>50%) than were patients with higher ECOG PS (45% vs. 32%, p=0.003). They also found that ECOG PS was a significant factor for time to PSA progression (p=0.043), OS (p<0.001), and PSA decline (p=0.002).
We also analyzed clinical variables according to ECOG PS. However, because of the small study size, we did not find a statistical difference between the groups, although we found a pattern similar to that in the previous study. Kaplan-Meier analysis demonstrated that the radiologic progression-free survival rate and clinical progression-free survival might be associated with ECOG PS.
Abiraterone acetate, a first-in-class agent, is a highly potent and tolerable androgen biosynthesis inhibitor that has been shown to elicit a median 4-month survival benefit in docetaxel-refractory patients [714]. Recently, abiraterone acetate has often been administered in men with mCRPC with a previous history of docetaxel failure. Hofner et al. [15] reported that progression-free survival with abiraterone acetate was significantly positively correlated with the duration of response to docetaxel chemotherapy. In addition, clinicopathologic factors such as high Gleason score, PSA decline, and low PSA nadir were reported to be predictive of response to abiraterone acetate [15].
Even though the efficacy of abiraterone after docetaxel failure has been established, the therapeutic benefit of targeting androgen receptor signaling by sequential administration of abiraterone is not clear. Noonan et al. [16] reported retrospective data showing that treatment with abiraterone was associated with a limited response rate and short duration of effect in patients experiencing progression after enzalutamide treatment.
Several mechanisms of intrinsic or acquired resistance to abiraterone treatment have been proposed in vitro and in vivo [17]. Ferraldeschi et al. [17] suggested elucidation of the mechanisms underlying resistance to abiraterone treatment. Potent and selective inhibition of CYP17A1 by abiraterone depletes residual nongonadal androgens and is an effective treatment for CRPC. Preclinical evidence that androgen biosynthesis in prostate cancer cells does not necessarily follow a single dominant pathway and that residual androgens or alternative ligands (including administered glucocorticoids) can reactivate androgen receptor signaling support the cotargeting of more than one enzyme involved in steroidogenesis and combining a CYP17A1 inhibitor with an antiandrogen. Furthermore, given the drawbacks of 17α-hydroxylase inhibition, there is considerable interest in developing new CYP17A1 inhibitors that more specifically inhibit lyase activity and are therefore less likely to require glucocorticoid coadministration.
Antonarakis et al. [18] found that androgen-receptor splice variant 7 messenger RNA (AR-V7) detection in circulating tumor cells from patients with mCRPC might be associated with resistance to abiraterone treatment. They found that AR-V7-positive patients had lower PSA response rates than did AR-V7-negative patients (0% vs. 68%, p=0.004) and shorter PSA progression-free survival (median, 1.3 months vs. not reached; p<0.001), clinical or radiographic progression-free survival (median, 2.3 months vs. not reached; p<0.001), and OS (median, 10.6 months vs. not reached, p=0.006). We also might need genetic information to confirm patients who show a good response to abiraterone treatment.
The limitations of our study include its retrospective nature and that the data were derived from a single institution. The small size of our cohort limits the generalization of our results. Concerning the statistical analysis, the relatively small sample size in our study could result in weak statistical power and generate the potential of bias, especially in multivariate analysis. Lack of genetic data for the patients administered abiraterone treatment limited our evaluation of the effect of previous ADT duration on the efficacy of abiraterone treatment after docetaxel failure.
Although this was a retrospective study with a small sample size, we did not observe any statistically significant differences in the clinical response to abiraterone acetate between mCRPC patients with a long ADT duration and those with a short ADT duration in terms of PSA progression-free survival, radiologic progression-free survival, and clinical progression-free survival. A large-scale, multicenter, prospective study is needed to fully evaluate the clinical effects of abiraterone acetate in patients with mCRPC according to previous ADT duration.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A1A2059537).
References
1. Agarwal N, Sonpavde G, Sternberg CN. Novel molecular targets for the therapy of castration-resistant prostate cancer. Eur Urol. 2012; 61:950–960. PMID: 22209376.

2. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014; 65:467–479. PMID: 24321502.

3. Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006; 24:3089–3094. PMID: 16809734.

4. Croke J, Leung E, Segal R, Malone S. Clinical benefits of alpharadin in castrate-chemotherapy-resistant prostate cancer: case report and literature review. BMJ Case Rep. 2012; 11. 01. 2012:pii: bcr2012006540. DOI: 10.1136/bcr-2012-006540.

5. Bahl A, Oudard S, Tombal B, Ozguroglu M, Hansen S, Kocak I, et al. Impact of cabazitaxel on 2-year survival and palliation of tumour-related pain in men with metastatic castration-resistant prostate cancer treated in the TROPIC trial. Ann Oncol. 2013; 24:2402–2408. PMID: 23723295.

6. Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol. 2008; 8:440–448. PMID: 18674639.

7. de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011; 364:1995–2005. PMID: 21612468.

8. Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013; 368:138–148. PMID: 23228172.

9. Clayton R, Heng DY, Wu JS, Zielinski R, North SA, Emmenegger U, et al. A multicenter population-based experience with abiraterone acetate (AA) in patients with metastatic castration resistant prostate cancer (mCRPC) [abstract]. J Clin Oncol. 2013; 31(Suppl 6):Abstract No. 113.
10. Halabi S, Lin CY, Small EJ, Armstrong AJ, Kaplan EB, Petrylak D, et al. Prognostic model predicting metastatic castration-resistant prostate cancer survival in men treated with second-line chemotherapy. J Natl Cancer Inst. 2013; 105:1729–1737. PMID: 24136890.

11. Chi KN, Kheoh TS, Ryan CJ, Molina A, Bellmunt J, Vogelzang NJ, et al. A prognostic model for predicting overall survival (OS) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) treated with abiraterone acetate (AA) after docetaxel [abstract]. J Clin Oncol. 2013; 31(Suppl):Abstract No. 5013.
12. Ryan CJ, Molina A, Li J, Kheoh T, Small EJ, Haqq CM, et al. Serum androgens as prognostic biomarkers in castration-resistant prostate cancer: results from an analysis of a randomized phase III trial. J Clin Oncol. 2013; 31:2791–2798. PMID: 23816964.

13. Azad AA, Eigl BJ, Leibowitz-Amit R, Lester R, Kollmannsberger C, Murray N, et al. Outcomes with abiraterone acetate in metastatic castration-resistant prostate cancer patients who have poor performance status. Eur Urol. 2015; 67:441–447. PMID: 24508071.

14. Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012; 13:983–992. PMID: 22995653.

15. Hofner T, Vallet S, Hadaschik BA, Pahernik S, Duensing S, Hohenfellner M, et al. Docetaxel followed by abiraterone in metastatic castration-resistant prostate cancer: efficacy and predictive parameters in a large single center cohort. World J Urol. 2015; 33:833–839. PMID: 25113804.

16. Noonan KL, North S, Bitting RL, Armstrong AJ, Ellard SL, Chi KN. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013; 24:1802–1807. PMID: 23585511.

17. Ferraldeschi R, Sharifi N, Auchus RJ, Attard G. Molecular pathways: inhibiting steroid biosynthesis in prostate cancer. Clin Cancer Res. 2013; 19:3353–3359. PMID: 23470964.

18. Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014; 371:1028–1038. PMID: 25184630.

Table 1
Baseline characteristics of the patients at the initiation of abiraterone treatment
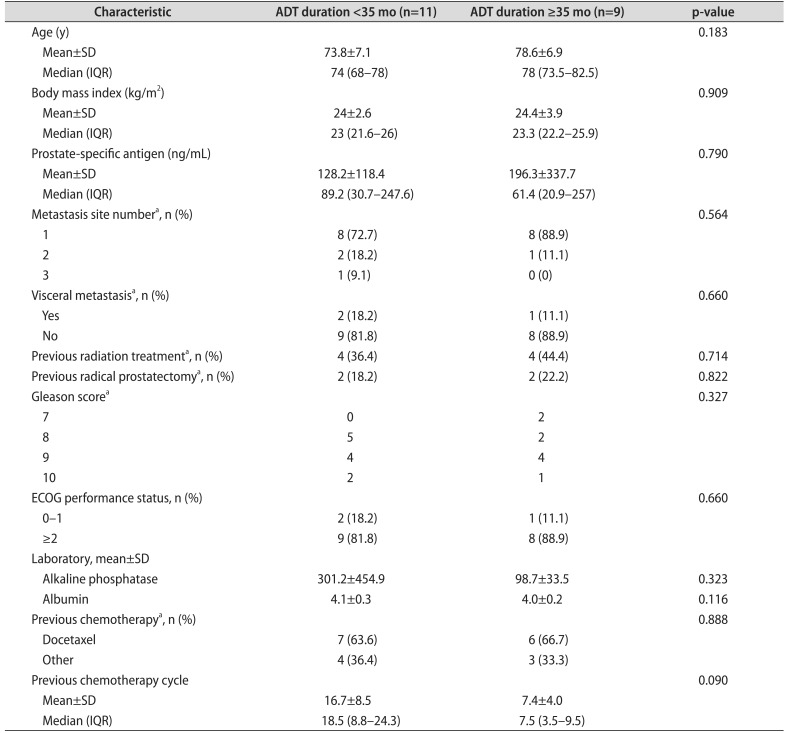
Table 2
Stratified clinical response to abiraterone according to duration of previous androgen deprivation therapy
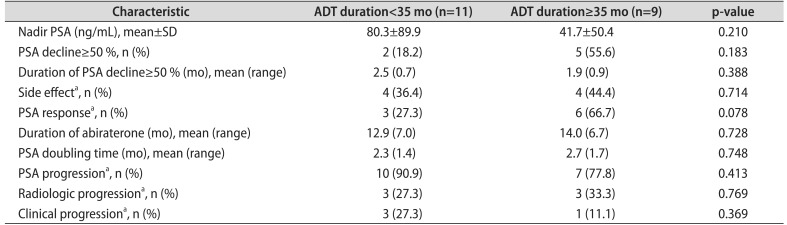
Table 3
Stratified clinical response to abiraterone in patients stratified by ECOG performance status




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download