Abstract
Purpose
To evaluate the clinical features and biochemical recurrence (BCR) in prostate cancer (PCa) with high-grade prostatic intraepithelial neoplasia (HGPIN).
Materials and Methods
We retrospectively analyzed the medical records of 893 patients who underwent a radical prostatectomy for PCa between 2011 and 2012 at Asan Medical Center; 752 of these patients who did not receive neoadjuvant or adjuvant therapy and were followed up for more than 1 year were included. The cohort was divided into two groups-patients with and without HGPIN-and their characteristics were compared. The Cox proportional hazards model was used to analyze factors affecting BCR.
Results
In total, 652 study patients (86.7%) had HGPIN. There were no significant differences in preoperative factors between the two groups, including age (p=0.369) and preoperative prostate-specific antigen concentration (p=0.234). Patients with HGPIN had a higher Gleason score (p=0.012), more frequent multiple tumor (p=0.013), and more perineural invasion (p=0.012), but no other postoperative pathologic characteristics were significantly different between the two groups. There were no significant differences in BCR (13.0% vs. 11.5%, p=0.665) and HGPIN was not associated with BCR (p=0.745). In multivariate analysis, only the T stage (p<0.001) was associated with BCR.
High-grade prostatic intraepithelial neoplasia (HGPIN), defined as architecturally benign prostatic acini and ducts lined by atypical cells, has similar histopathological characteristics to prostate adenocarcinoma [1]. HGPIN is known as a precancerous lesion of prostate cancer (PCa) [2]. Recent studies recommended repeat biopsy after initial diagnosis of HGPIN. The risk of cancer following a diagnosis of isolated HGPIN is about 31.5%, although up to 10% of men with a diagnosis of HGPIN will not have a diagnosis of cancer until the third or fourth biopsy [34]. Furthermore, an initial diagnosis of HGPIN before a later diagnosis of PCa does not increase the risk of adverse pathologic features and is associated with older age, lower grade, and smaller tumor volume [56].
Praecancerous lesions such as carcinoma in situ of the bladder, lobular carcinoma in situ of the breast, and intraepithelial neoplasia of the cervix are associated with adverse clinical outcomes [789]. However, recent studies have sparked a debate on the value of prostatic intraepithelial neoplasia as a prognostic factor for PCa [3610111213]. HGPIN simultaneously presents with prostate adenocarcinoma in up to 88.4% of radical prostatectomy specimens. However, too few studies have been conducted to adequately evaluate the impact of HGPIN on the clinicopathological features of PCa. Furthermore, there have been no studies of the relationship between concomitant HGPIN and clinical outcomes of PCa. Additionally, the value of concomitant HGPIN as a prognostic factor in PCa following a prostatectomy is still under debate [14151617].
Our present study investigated the association between the clinicopathological features of PCa with concomitant HGPIN in a series of prostatectomy specimens and evaluated the prognostic value of HGPIN in PCa.
The presence of HGPIN has been systematically recorded since March 2011 at our institution, Asan Medical Center. We retrospectively analyzed the medical records of 893 patients who underwent radical prostatectomy for PCa at our center from March 2011 to December 2012. Operation methods included retropubic radical prostatectomy and robot-assisted laparoscopic prostatectomy. Of these patients, we enrolled the 752 cases that did not receive neoadjuvant or adjuvant therapy and had a follow-up period of more than 1 year. This retrospective study was approved by our institutional review board.
The preoperative and postoperative variables assessed in our current analyses included age, preoperative prostate-specific antigen (PSA), body mass index (BMI), pathological Gleason score, presence of lymphovascular invasion (LVI) and perineural invasion (PNI), presence of HGPIN, prostate volume, percent tumor volume (PTV), tumor multiplicity, extracapsular extension, seminal vesicle invasion, presence of positive surgical margin, and biochemical recurrence (BCR). The definition of BCR is a PSA level greater than 0.2 ng/mL on two consecutive measurements. All of the study patients had been preoperatively staged for metastases using contrast-enhanced magnetic resonance imaging and bone scintigraphy. The patients were divided into three groups according to their pathological Gleason scores, and subgroup analyses were performed to investigate the clinicopathological features of these groups.
The 2010 American Joint Committee on Cancer/TNM staging system and the Gleason system were used for pathologic staging and tumor grading [18]. PNI was defined as tumor tracking along or around the nerve sheath. LVI was defined as an unequivocal presence of tumor cells in an endothelium-lined space. HGPIN was defined as a pathological feature characterized by neoplastic transformation of the secretory epithelium lining, prostatic ducts, and acini [19202122]. All pathological diagnoses were made by uropathologists at our institution. We retrospectively collected the pathologic data by reviewing pathologic reports.
Statistical analyses were performed to compare the clinical features and outcomes with the presence of HGPIN. The Student t-test was used for quantitative variables, whereas chi-square or Fisher exact tests were used for qualitative variables. The Mann-Whitney U test was used for nonparametric variables. The Cox proportional hazard regression model was used to predict the significance of the clinicopathological variables for BCR. All statistical tests were two-sided with p<0.05 considered statistically significant. Only variables determined to be statistically significant by univariate analysis (p<0.2) were included in the multivariate analysis. All analyses were performed with PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA).
The characteristics of the PCa patients in the study cohort with and without HGPIN are listed in Table 1. Patients with HGPIN comprised 86.7% of the study population. The mean age was 66 years, mean BMI was 24.6 kg/m2, mean preoperative PSA was 9.3 ng/mL, mean prostate volume was 40.9 mL, and mean tumor percentage was 15%. Patients without HGPIN were more likely to have a Gleason score of 6 or less (30.0% vs. 17.9%) and less likely to have a Gleason score of 7 (53.0% vs. 66.1%). The percentage of patients with a Gleason score of 8 or greater did not differ significantly between the two groups (17.0% vs. 16.0%).
PNI was seen in 53.0% of the group without HGPIN and 66.0% of the group with HGPIN (p=0.012). Additionally, 66% of the group without HGPIN and 77.5% of the group with HGPIN had multiple tumors (p=0.013). There were no significant differences found between the two groups in any of the other variables analyzed, including pathologic T stage, PSA, presence of LVI, and lymph node involvement. The median follow-up period was 28.9 months. BCR occurred in 11.7% of patients during follow-up-13.8% in the group without HGPIN and 11.2% in the group with HGPIN (p=0.665). There was no significant difference in the BCR rates between the two groups.
The results of univariate and multivariate analyses of the association of clinicopathological variables with BCR are summarized in Table 2. In the univariate analysis, preoperative PSA, pathological T stage, pathological Gleason score, presence of PNI, presence of LVI, lymph node involvement, positive surgical margin, and PTV were found to be significant predictors of BCR. In the multivariate analysis, only the pathological T stage was found to be an independent predictor of BCR (p<0.001). HGPIN showed no association with BCR by either univariate or multivariate analysis (Fig. 1).
Subgroup analyses were performed for each patient group divided according to the Gleason score (Table 3). In the Gleason score≤6 and Gleason score 7 groups, there were no differences in most of the clinical and pathological variables between patients with and without HGPIN. In the Gleason score≤6 group, patients with HGPIN had a higher PTV than those without HGPIN (mean±standard deviation [SD], 6.1±6.3 vs. 3.4±2.4, p=0.012). However, in the Gleason score 8 group (n=121), there was no significant difference in the percentage tumor volume between the two groups (mean±SD, 25.2±22.6 vs. 44.1±37.0, p=0.095). High Gleason score patients with HGPIN had multiple tumors more often than those without HGPIN (78.8% vs. 47.1%, p=0.005).
In 1986, McNeal and Bostwick [23] classified these lesions into mild, moderate, and severe dysplasia, but a simplified two-grade classification has since been recommended. In 1987, Bostwick and Brawer [1] defined prostatic intraepithelial neoplasia as a precursor of PCa, which is characterized by changes similar to tumorous prostate gland cells but differs from adenocarcinomas in that the basal cell layer and basement membrane are intact. However, it remains unclear which HGPIN foci will develop into PCa.
The role of HGPIN as a prognostic factor for PCa after a prostatectomy has remained uncertain. Qian et al. [24] found a correlation between the total volume of concomitant HGPIN and the volume, stage, and differentiation degree of PCa, which supports the contention that HGPIN is associated with poorer clinical outcomes and the histological characteristics of PCa. Pierorazio et al. [14] also found that PNI and multiplicity more frequently presented in the prostatectomy specimens of patients with HGPIN. These authors reported that BCR-free survival was better in patients without HGPIN, and that the risk of BCR was higher in patients with HGPIN. Auskalnis et al. [25] found that patients with HGPIN showed poorer histopathological characteristics, such as a higher Gleason score and TNM stage, but that HGPIN had no influence on the BCR of the PCa. In another study by Ingels et al. [16], HGPIN was associated with better biological and pathological features and with better BCR-free survival by Kaplan-Meier regression, but there were no significant associations found by multivariate analysis. Sfoungaristos and Perimenis [26] suggested that the presence of concomitant HGPIN and PCa are not related to tumor aggressiveness in PCa patients undergoing a radical prostatectomy and should not be considered as parameters for operative outcome prediction.
In our present study, we investigated the clinicopathological features of a PCa cohort in relation to the presence of HGPIN and the influence of HGPIN on BCR outcomes. Patients with HGPIN tended to have a higher Gleason score, more PNI, and more frequent multiple tumors. Furthermore, the presence of HGPIN was not found to be an independent predictor of BCR after a radical prostatectomy when analyzed using a Cox proportional hazard regression model. In our subgroup analysis based on a Gleason score stratification, the PTV was higher in Gleason score 6 patients with HGPIN than without HGPIN. In the Gleason score≥8 groups, the HGPIN cases had multiple tumors more often than the patients without HGPIN.
In our current study, the presence of HGPIN was found to be associated with tumor focality. However, previous studies have reported that tumor focality is not associated with the prognosis of localized PCa [2728]. Although there are no reported clinical implications from recent studies, the association of HGPIN with tumor focality was clear from our current analyses and might represent a meaningful result regarding the characteristics of HGPIN.
Based on the cumulative evidence to date, including our current findings, we hypothesize that PCa derived from HGPIN is more likely to progress to high-grade PCa than "de novo" PCa. Before becoming a malignant lesion, HGPIN likely expands to the wider prostate and gradually progress to malignancy. Extended malignant lesions might have more opportunities for aggravation. Similarly, Pierorazio et al. [14] suggested that HGPIN is a negative prognostic indicator for PCa. These authors hypothesized that the field defect is in the entire gland, leading to more diffuse disease and potentially greater dedifferentiation of adenocarcinoma cells prior to diagnosis. However, PCa derived from HGPIN would have a more indolent oncogenic course than "de novo" PCa. "De novo" PCa does not develop from a premalignant lesion, is infiltrative, and grows rapidly. Analogously, Ingels et al. [16] suggested that the presence of HGPIN is associated with fewer serious biological and pathological features and a better BCR-free survival outcome. These authors interpreted that HGPIN is a sign of slower oncogenesis and a longer duration of premalignancy before carcinoma development.
In our current subgroup analysis, the PTV was higher for the Gleason score 6 patients with HGPIN and the Gleason score≥8 patients without HGPIN. Although this apparently conflicting result is difficult to interpret due to the small sample size in our analyses, we speculate that PCas derived from HGPIN have an extended premalignancy stage and therefore a higher chance of progression to a low-grade tumor, thus yielding patients with low-grade tumors that have a higher percent volume. However, in cases of high-grade tumors, "de novo" PCas have a more aggressive behavior, so they grow rapidly and have a higher PTV.
PNI has emerged as a prognostic indicator in many different malignancies. In the urologic field, PNI is evident in 31.9%-79.0% of prostate adenocarcinomas [29]. The role of PNI as a prognostic factor in PCa is controversial, but many recent studies have suggested that PNI is a predictor of a poorer clinical outcome for patients with this disease [1530].
This study had some notable limitations. The follow-up period was relatively short, and the occurrence of BCR was below the threshold required for proper statistical analysis. In our center, prostatic intraepithelial neoplasia in prostatectomy specimens has been described regularly since March 2011. In our present study, BCR occurred in 88 patients, 15 with stage T2 or less lesions and 73 with stage T3 or greater cancers. Most instances of BCR occurred in patients with higher pathological stage tumors and higher Gleason score tumors. Hence, certain factors, including the T stage and Gleason score, would have a greater influence on the results of multivariate analysis than others. The prognostic value of established markers did not exactly match with known data. For example, the pathologic Gleason score was not found to be a significant predictor of BCR by multivariate analysis in our current study. Exclusion of high-risk patients who received neoadjuvant or adjuvant therapy and short follow-up periods might have affected the power of the prognostic variables. There is also the possibility of error in our interpretation of the pathological findings reported herein. In our study of patients with high-grade disease, the tumors had aggressive and rapid growing features, meaning that a high tumor volume could have replaced the HGPIN, thereby mimicking "de novo" PCa. We could not demonstrate with statistical significance that the presence of HGPIN affected BCR. The presence of HGPIN may explain the adverse pathological findings for the prostatectomy specimens. However, as the definite treatment, a radical operation might have affected the clinical outcome, so the clinical impact of HGPIN might not have been revealed. We used pathological findings of prostatectomy specimens, so the subjectivity of the pathologic reports is an important consideration. However, the clinical implications of HGPIN are controversial, so detailed information about HGPIN was not described in the pathologic report. Furthermore, it is possible that the presence of a premalignant lesion might be ignored in specimens of known malignancy. Further studies that include a systemic review of pathological specimens are needed.
These limitations notwithstanding, our present study reports an association between HGPIN and adverse pathological findings in PCa patients, including Gleason score and perineural invasion. Furthermore, patients with HGPIN were more likely to have multifocal tumors than those without HGPIN. This finding supports our hypothesis that PCa derived from HGPIN will spread further and progress more quickly to a more diffuse malignant lesion.
Considering our current study findings and recent studies that have reported similar results, PCa with concomitant HGPIN should be carefully observed postoperatively and may require further management. Even though we did not find a significant link between HGPIN and PCa outcomes in our present analyses, we believe that this single-center study will help to fully elucidate the role of HGPIN in PCa in the future. Further large, long-term follow-up cohort investigations are essential to clarify the role of HGPIN as a predictor of adverse outcomes in PCa patients.
Figures and Tables
Fig. 1
Comparison of biochemical recurrence (BCR)-free survival outcomes between prostate cancer patients with or without concomitant high grade prostatic intraepithelial neoplasia (HGPIN).
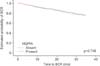
Table 1
Patient characteristics

Table 2
Univariate and multivariate analyses of biochemical recurrence in the study cohort

Table 3
Subgroup analysis of the clinicopathological features of study groups stratified by the Gleason score

ACKNOWLEDGMENTS
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI06C0868).
References
1. Bostwick DG, Brawer MK. Prostatic intra-epithelial neoplasia and early invasion in prostate cancer. Cancer. 1987; 59:788–794.
2. Haggman MJ, Macoska JA, Wojno KJ, Oesterling JE. The relationship between prostatic intraepithelial neoplasia and prostate cancer: critical issues. J Urol. 1997; 158:12–22.
3. Epstein JI, Herawi M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol. 2006; 175(3 Pt 1):820–834.
4. Herawi M, Kahane H, Cavallo C, Epstein JI. Risk of prostate cancer on first re-biopsy within 1 year following a diagnosis of high grade prostatic intraepithelial neoplasia is related to the number of cores sampled. J Urol. 2006; 175:121–124.
5. Al-Hussain TO, Epstein JI. Initial high-grade prostatic intraepithelial neoplasia with carcinoma on subsequent prostate needle biopsy: findings at radical prostatectomy. Am J Surg Pathol. 2011; 35:1165–1167.
6. Pietzak EJ 3rd, Kabarriti AE, Mucksavage P, Bavaria T, Van Arsdalen K, Malkowicz SB, et al. The presence of high-grade prostatic intraepithelial neoplasia or atypia on prostate biopsy does not adversely affect prostatectomy outcomes for patients otherwise eligible for active surveillance. Urology. 2014; 84:1442–1447.
7. Casey RG, Catto JW, Cheng L, Cookson MS, Herr H, Shariat S, et al. Diagnosis and management of urothelial carcinoma in situ of the lower urinary tract: a systematic review. Eur Urol. 2015; 67:876–888.
8. Horst KC, Smitt MC, Goffinet DR, Carlson RW. Predictors of local recurrence after breast-conservation therapy. Clin Breast Cancer. 2005; 5:425–438.
9. WHO Guidelines for treatment of cervical intraepithelial neoplasia 2-3 and adenocarcinoma in situ: cryotherapy, large loop excision of the transformation zone, and cold knife conization. Geneva: World Health Organization;2014.
10. Moore CK, Karikehalli S, Nazeer T, Fisher HA, Kaufman RP Jr, Mian BM. Prognostic significance of high grade prostatic intraepithelial neoplasia and atypical small acinar proliferation in the contemporary era. J Urol. 2005; 173:70–72.
11. Thompson IM Jr, Leach R. Prostate cancer and prostatic intraepithelial neoplasia: true, true, and unrelated? J Clin Oncol. 2013; 31:515–516.
12. Humphrey PA. High grade prostatic intraepithelial neoplasia in prostate needle biopsy. J Urol. 2013; 189:315–316.
13. Gallo F, Chiono L, Gastaldi E, Venturino E, Giberti C. Prognostic significance of high-grade prostatic intraepithelial neoplasia (HGPIN): risk of prostatic cancer on repeat biopsies. Urology. 2008; 72:628–632.
14. Pierorazio PM, Lambert SM, Matsukhani M, Sprenkle PC, McCann TR, Katz AE, et al. High-grade prostatic intraepithelial neoplasia is an independent predictor of outcome after radical prostatectomy. BJU Int. 2007; 100:1066–1070.
15. Jung JH, Lee JW, Arkoncel FR, Cho NH, Yusoff NA, Kim KJ, et al. Significance of perineural invasion, lymphovascular invasion, and high-grade prostatic intraepithelial neoplasia in robot-assisted laparoscopic radical prostatectomy. Ann Surg Oncol. 2011; 18:3828–3832.
16. Ingels A, Ploussard G, Allory Y, Abbou C, de la Taille A, Salomon L. Concomitant high-grade prostatic intraepithelial neoplasia is associated with good prognosis factors and oncologic outcome after radical prostatectomy. Urol Int. 2014; 92:264–269.
17. López JI. Prostate adenocarcinoma detected after high-grade prostatic intraepithelial neoplasia or atypical small acinar proliferation. BJU Int. 2007; 100:1272–1276.
18. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer;2010.
19. Epstein JI, Srigley J, Grignon D, Humphrey P. Association of Directors of Anatomic and Surgical Pathology. Recommendations for the reporting of prostate carcinoma: Association of Directors of Anatomic and Surgical Pathology. Am J Clin Pathol. 2008; 129:24–30.
20. Recommendations for the reporting of resected prostate carcinomas. Association of Directors of Anatomic and Surgical Pathology Agency. Pathol Int. 1997; 47:268–271.
21. Srigley JR, Amin MB, Epstein JI, Grignon DJ, Humphrey PA, Renshaw AA, et al. Updated protocol for the examination of specimens from patients with carcinomas of the prostate gland. Arch Pathol Lab Med. 2006; 130:936–946.
22. Bostwick DG, Liu L, Brawer MK, Qian J. High-grade prostatic intraepithelial neoplasia. Rev Urol. 2004; 6:171–179.
23. McNeal JE, Bostwick DG. Intraductal dysplasia: a premalignant lesion of the prostate. Hum Pathol. 1986; 17:64–71.
24. Qian J, Wollan P, Bostwick DG. The extent and multicentricity of high-grade prostatic intraepithelial neoplasia in clinically localized prostatic adenocarcinoma. Hum Pathol. 1997; 28:143–148.
25. Auskalnis S, Milonas D, Jievaltas M, Vaiciunas K, Mickevicius A, Gudinaviciene I. The role of high-grade prostatic intraepithelial neoplasia for biochemical relapse of prostate carcinoma after radical prostatectomy. Medicina (Kaunas). 2010; 46:604–610.
26. Sfoungaristos S, Perimenis P. Implication of high grade intraepithelial neoplasia in adverse pathology after radical prostatectomy. Prague Med Rep. 2012; 113:156–165.
27. Masterson TA, Cheng L, Mehan RM, Koch MO. Tumor focality does not predict biochemical recurrence after radical prostatectomy in men with clinically localized prostate cancer. J Urol. 2011; 186:506–510.
28. Iremashvili V, Pelaez L, Manoharan M, Acosta K, Rosenberg DL, Soloway MS. Tumor focality is not associated with biochemical outcome after radical prostatectomy. Prostate. 2012; 72:762–768.
29. Tollefson MK, Karnes RJ, Kwon ED, Lohse CM, Rangel LJ, Mynderse LA, et al. Prostate cancer Ki-67 (MIB-1) expression, perineural invasion, and gleason score as biopsy-based predictors of prostate cancer mortality: the Mayo model. Mayo Clin Proc. 2014; 89:308–318.
30. Lee JT, Lee S, Yun CJ, Jeon BJ, Kim JM, Ha HK, et al. Prediction of perineural invasion and its prognostic value in patients with prostate cancer. Korean J Urol. 2010; 51:745–751.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download