Abstract
Purpose
Heat shock protein (HSP) 27 protects the cell by controlling apoptosis and immune reactions, and c-FLIP (cellular-FLICE inhibitory protein) inhibits apoptosis by inhibiting caspase-8 activity. We investigated the relationship of HSP27 and c-FLIP expression to prostate-specific antigen, Gleason score sum (GSS), and pathologic stage.
Materials and Methods
Samples from 163 patients between May 2004 and April 2010 were analyzed: 83 from patients that had underwent a radical prostatectomy, and 80 from those that underwent transurethral resection of the prostate to alleviate urinary symptoms from benign prostate hyperplasia. c-FLIP and HSP27 expression were observed by immunohistochemistry staining. Samples with less than 5% expression-positive cells were scored as 1, with 5%-50% were scored as 2, and with more than 50% were scored as 3. Local reactions were identified as 0.5 and evaluated.
Results
Both the presence of HSP27 within the tumor and the number of cancer cells positive for HSP27 were significantly correlated to GSS and pathologic stage (p<0.001, p=0.001, p<0.001, p<0.001). The same was true for c-FLIP expression (p<0.001). GSS was more highly correlated to HSP27 expression than to c-FLIP expression (r=0.814 for HSP27, r=0.776 for c-FLIP), as was pathologic stage (r=0.592 for HSP27, r=0.554 for c-FLIP).
Conclusions
In prostate cancer, higher GSS and a more advanced pathologic stage were associated with a higher likelihood of having a HSP27-positive tumor and more HSP27-positive tumor cells. HSP27 expression was correlated with GSS and prostate cancer stage. A more advanced pathologic stage corresponded to a higher likelihood of having a c-FLIP-positive tumor and more c-FLIP-positive tumor cells. HSP27 expression had a higher correlation with prostate cancer stage and GSS than c-FLIP expression did.
Prostate cancer is increasingly prevalent in Korea, where the Western lifestyle prevails. A study of the relationship between the pathologic stages of prostate cancer and the Gleason score sum (GSS) is important for refining prostate cancer diagnosis and treatment.
Among the antiapoptotic factors, bcl-2, clusterin, heat shock protein (HSP) 27, cellular-FLICE inhibitory protein (c-FLIP), and glucose-related protein 78 (GRP78) have been most extensively studied. Expression of HSP27 plays an important role in prostate cancer [1]. HSP assists the folding, activation, trafficking, and transcriptional activities of most steroid receptors, including the androgen receptor (AR). HSP27 also plays important roles in apoptosis signaling related to caspase 3. HSP27 interrupts the cytochrome c secretion of thread granules in the procaspase 9 pathway. It also acts on cytochrome c or procaspase 3 to inhibit apoptosome formation and apoptosis [2]. One study demonstrated that HSP expression inhibits apoptosis, and other studies have found that HSP27 is related to the acquisition of hormone resistance in the Lymph node of prostate cancer cell line, a type of prostate cancer [3]. c-FLIP, an important protein in prostate cancer, inhibits apoptosis mediated by Fas within death-inducing signal complexes by inhibiting caspase-8 activity [4]. c-FLIP is not expressed in normal bladder cells, and higher c-FLIP expression is associated with a more advanced disease stage [5]. In addition, c-FLIP expression increases in stomach and gallbladder cancers [6].
Dual silencing decreases proliferation and enhances apoptosis of cancer cells in the laboratory and is technically feasible [7]. Because HSP27, one of the small Hsps, inhibits key effectors of the apoptotic pathway at the pre- and postmitochondria levels and because c-FLIP is regarded as a new therapeutic target for relevant cancers, we investigated these two factors to quantify their relationship with GSS and pathologic state [89]. In prostate cancer, HSP27 is correlated with pathologic stage, Gleason score, lymph node metastasis, shorter the time until biochemical recurrence (BCR), and poor clinical outcome [1011].
Previous studies report separate correlations for HSP27 expression and c-FLIP expression to clinical features in cancers, including prostate cancer [14]. However, there is little knowledge about the impact of simultaneous expression of these two proteins with the levels of correlation. According to a recent study by Kim et al. [12], the AR is correlated with increased expression of cancerrelated survival markers, such as HSP27, GRP78, clusterin, and c-FLIP, in prostate cancer. Those authors used androgensensitive human prostate cancer cells derived from a lymph node metastasis.
c-FLIP directly participates in c-Jun N-terminal protein kinase (JNK) activation, a key activity that regulates cellular events, through Mitogen-activated protein kinase kinase 7 (MKK7) in a tumor necrosis factor-α-dependent manner. The interaction of c-FLIPL (the long form of c-FLIP) with MKK7 might selectively suppress JNK activation [13]. HSP27 increases tumor activation though upstream serial signaling with MKK4 (MEK4), which could result in HSP27-mediated increases in invasion with the phosphorylation of HSP27 [1415]. Considering the emerging evidence about the functional role of MKK-4, MKK-6, and MKK-7 in prostate cancer [16] and the fact that we could not assay the direct relationship of MKK with prostate cancer, we designed a pioneer experiment with HSP27 and c-FLIP that could hint at its role.
Moreover, there is little knowledge about how the simultaneous expression of HSP27 and c-FLIP relates to clinical variables. We used an immunohistochemical method to identify correlations between HSP27 and c-FLIP expression with GSS and pathologic stage by analyzing tissues obtained after prostate cancer surgery.
This study included 163 patients diagnosed with prostate cancer and benign prostate hyperplasia (BPH). Between May 2004 and April 2010, 83 patients had a radical prostatectomy (n=81) and transurethral resection (n=2) who could not under gone prostatectomy. As a control, we used samples from 80 patients who had undergone transurethral resection of the prostate to alleviate urinary symptoms from BPH. Patient age, tumor stage, tumor GSS, presence or absence of lymph node metastasis, and presence or absence of distant organ metastasis were investigated. Pathologic stages were classified according to the clinical TNM stages, revised in 2002.
After the tissues were obtained from surgery or biopsy, they were fixed in 10% neutral buffer formalin and serial sections of 5 µm in thickness were embedded in paraffin. These serial sections were attached to slides, dried, and then processed through deparaffin and hydrous procedures. The paraffin-embedded samples were then treated with 3% hydrogen peroxide for 10 minutes to prevent peroxidase activation. These were heated for 10 minutes in a pressure apparatus containing 10mM citrate buffer (pH 6.0). After cleaning three times for 2 minutes with phosphate-buffered saline (PBS) to prevent abnormal binding of the background, they were pretreated with blocking solution for 10 minutes. A mouse monoclonal antibody to HSP27 was purchased from Leica Microsystems (NCL-HSP27 Leica Microsystems Newcastle Ltd., Newcastle Upon Tyne, UK). For c-FLIP immunohistochemistry, polyclonal anti-c-FLIP antibody (1:50 dilution; Enzo Life Sciences, Philadelphia, PA, USA) was used.
After adding the primary antibodies to HSP27 and c-FLIP, samples were treated for 1 hour at room temperature and then cleaned with Tris buffer. Samples were treated with biotinylated secondary antibody and streptavidin-HRP conjugate (Dako LSAB Kit, K0675, Carpinteria, CA, USA) for 10 minutes each and then cleaned with Tris buffer. 3,3'-diaminobenzidine (Sigma Chemical, St. Louis, MO, USA) color fixing and Mayer's hematoxylin contra staining were performed. Slides were stained on an automated immunostainer (DakoCytomation, Glostrup, Denmark). As a negative control, the primary antibody was omitted and replaced with PBS. The immunohistochemical staining results of HSP27 and c-FLIP demonstrated the expression pattern in tumor cell cytosol. If a local reaction abnormality took place, it was judged as positive, and positive cell numbers were counted under ×100 magnification microscopy [17]. And the slides were scored by single pathologist. After the procedure was repeated three times to compute an average, the results were analyzed by counting positive expression levels. Positive cell numbers below 5% were denoted as +1, between 5%-50% as +2, and above 50% as +3; this scale is referred to as the reaction score. Local reactions were denoted as +0.5.
The Institutional Review Board of Eulji University Hospital approved this study (IRB No. EMC 15-56).
The chi-square test was used to analyze HSP27- and c-FLIP-positive reaction rates, GSS, and pathologic stage in prostate cancer cells. Analysis of variance was used for correlation analysis of the average reaction scores of HSP27 and c-FLIP, GSS, and pathologic stages. A Duncan test was used to compare multiple groups. Chi-square test was used to analyze the correlation between HSP27 and c-FLIP expression, GSS, and pathologic stage. Logistic regression analyses were performed to analyze the association of each clinical variable with expression of HSP27 and c-FLIP. The statistical software program PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
The average patient age was 69.58±7.52, body mass index (BMI) was 26.4±6.54 kg/m2, and prostate volume was 42.23±7.54 mL. Two cases (2.41%) were stage T1, 44 cases (53.01%) were T2, 26 cases (31.33%) were T3, and 11 cases (13.25%) were T4. For the GSS, three cases (3.61%) had a GSS of 4 points, four cases (4.82%) had a GSS of 5 points, eight cases (9.64%) had a GSS of 6 points, 25 cases (30.12%) had a GSS of 7 points, 16 cases (19.28%) had a GSS of 8 points, and 24 cases (28.92%) had a GSS of 9 points. Lymph node metastasis was observed in nine cases (10.84%), and remote metastasis was observed in two cases (2.41%) (Table 1).
The group of BPH had 67.48±6.72 in age, BMI was 27.2±7.01 kg/m2, and prostate volume was 51.47±8.59 mL. Immunohistochemistry of C-FLIP and HSP27 were negative in BPH group.
There was no HSP27 positive reaction in three cases with a GSS of 4 points, in one case with a GSS of five points (25%), in one case with a GSS of six points (12.5%), in 15 cases with a GSS of seven points (60%), and in all 16 cases with a GSS of eight points. However, 23 of 24 cases with a GSS of nine points (95.83%) and all three cases with a GSS of 10 points had HSP27-positive reactions (p<0.001). Positive reactions were observed in one of two stage T1 cases (50%), 25 out of 44 T2 cases (56.82%), 23 out of 26 T3 cases (88.46%), and 10 out of 11 T4 cases (13.25%) (p=0.001) (Fig. 1).
A c-FLIP-positive reaction was not observed in three cases with a GSS of 4 points and in four cases with a GSS of 5 points. It was observed in three out of eight cases (37.5%) with a GSS of 6 points, 22 out of 25 cases (88%) with a GSS of 7 points, 15 out of 16 cases (93.75%) with a GSS of 8 points, all 24 cases with a GSS of 9 points, and all three cases with a GSS of 10 points (p<0.001) (Fig. 2). Overall, reactions were observed in one of two T1 cases (50%), 29 out of 44 T2 cases (65.91%), all 26 T3 cases, and all 11 T4 cases (p<0.001) (Table 2).
The averages in each HSP27 reaction range were 0.25, 0.13, 0.82, 2.13, 2.75, and 3 with a GSS of 4, 5, 6, 7, 8, 9, and 10 points, respectively (p<0.001), and 1, 1.01, 2.15, and 2.64 in stages T1, T2, T3, and T4 (p<0.001), respectively (Table 3).
In a multiple group post hoc comparison, the average HSP27 reaction score increased in proportion to GSS between 4 and 10 points. It also increased from stage T1 through T4. HSP27 was expressed in all nine cases with lymph node metastasis and 26 of 74 cases (53%) without lymph node metastasis. There was a statistically significant relationship between lymph node metastasis and the expression pattern of HSP27 (p<0.001). All patients with remote organ metastasis had HSP27 positive reactions. HSP27 expression was not observed in the BPH tissue selected as the control group. There was no correlation between the proportion of HSP27 positive reactions or reaction score to age. In logistic regression, HSP 27 expression was related with prostate volume, PSA level, biopsy Gleason score and percentage of maximum core involvement (Table 4).
The averages in each c-FLIP reaction range were 0, 0, 0.63, 1.08, 1.44, 2.33, and 3 with a GSS of 4, 5, 6, 7, 8, 9, and 10 points (p<0.001), respectively, and 1, 0.95, 1.92, and 2.36 with stages T1, T2, T3, and T4 (p<0.001), respectively (Table 3). In multiple group post hoc comparison, the c-FLIP average reaction score increased in proportion to GSS between a GSS of 4 and 10 points. It also increased with stage from T1 through T4. c-FLIP was expressed in all nine cases with lymph node metastasis and 26 of 74 cases (53%) without lymph node metastasis. There was a statistically significant relationship between lymph node metastasis and c-FLIP expression pattern (p<0.001). All patients with remote organ metastasis had c-FLIP-positive reactions. c-FLIP expression was not observed in the BPH tissue selected as the control group. There was no significant relationship between the proportion of c-FLIP positive reactions or reaction score to age. In logistic regression, c-FLIP expression was also related with prostate volume, PSA level, biopsy Gleason score and percentage of maximum core involvement (Table 5).
An important protein in cell biology, HSP was well preserved during evolution. Its production is initiated by various stressful stimuli including heat, ultraviolet irradiation, heavy metals, infection, inflammation, hypoxia, tissue damage, and tumors. HSP27 controls dynamic changes in actin and apoptosis. HSP27 reacts to the cytochrome c/Apaf-1/dATP complex in the procaspase 9 pathway and partially inhibits connectivity between Fas, Ask1, and daxx proteins [18]. In addition, HSP27 inhibits cytochrome c secretion in thread granules of the procaspase 9 pathway. HSP27 also inhibits caspase 3 activation and apoptosome formation by reacting to cytochrome c and procaspase 3 [19]. In this study, HSP27 responded strongly to immunohistochemical staining in prostate cancer cell lines.
HSP initiates danger signals by reacting to antigenic chaperone peptides in tumor cells and by inducing immune reactions that protect tumor cells, as evidenced by formation of HSP-peptide complexes from isolated dead tumor cells by antigen-presenting cells. Additionally, HSP aids in cytotoxic T-lymphocyte recognition by improving the ability to treat and transmit antigens in tumor cells [20]. Based on this, it is understood that HSP27 is expressed in prostate cancer cell lines. In this study, HSP27 expression differed depending on GSS and pathologic stage.
Miyake et al. [21] reported that while HSP27, HSP70, and HSP90 were present in prostate cancer cells, only expression of HSP27 was significantly correlated with Gleason score and pathologic stage. They also reported that HSP27 expression is useful predictor of BCR in prostate cancer. Similar to previous studies, this study also demonstrated the relationship between HSP27 with GSS and pathologic stage. HSP27 was more highly correlated than c-FLIP with GSS and pathologic stage.
Rocchi et al. [3] reported that the increase in HSP27 protected cells from hormone-refractory prostate cancer development after hormone treatment. They also reported that HSP27 played a role in controlling apoptosis. Teimourian et al. [22] reported that viability significantly decreased in prostate cancer cell lines after 72 hours of irradiation and had decreased HSP27 expression compared to that of other prostate cancer cell lines. In the present study, HSP27 was expressed in prostate cancer. Higher HSP27 expression was correlated with higher GSS and more advanced pathologic stage.
Day et al. [8] observed c-FLIP knockdown in Michigan cancer foundation-7 breast cancer cells, reporting that the knockdown of c-FLIP with siRNA transfection triggered spontaneous apoptosis and induced FAS-associated death domain-mediated and D related-5-mediated apoptosis. They assessed c-FLIPL, not c-FLIPS, as having a key role in preventing spontaneous death signaling and suggested c-FLIPL as a therapeutic target for breast cancer [23]. Similarly, on colorectal cancer cells, Longley et al. [24] reported that siRNA targeting c-FLIPL synergistically enhanced chemotherapy-induced apoptosis.
Survival markers including HSP27 and c-FLIP could be important for clarifying the relationship of JNK activation with apoptosis in prostate cancer. In our study, HSP27 expression was correlated with GSS and prostate cancer stage. More advanced pathologic stages corresponded to a higher incidence of c-FLIP presence and expression.
c-FLIP is critical to prostate cancer progression because it inhibits apoptosis mediated by caspase-8 [25]. In this study, c-FLIP was correlated with GSS and pathologic stage, indicating that the level of c-FLIP expression plays a major role in cancer progress.
Activated cytoplasm is required for tumor cell survival, and c-FLIP plays an important role in inhibiting apoptosis. In prostate cell cytoplasm, the level of c-FLIP expression is correlated to proliferation and infiltration in vitro [26]. c-FLIP contributes to the resistance of metastatic prostate cancer cells to docetaxel, tumor necrosis factor-related apoptosis-inducing ligand, and oxaliplatin. This indicates that c-FLIP has potential value as a resistance marker during medical treatment for metastatic prostate cancer [27]. Overexpression of c-FLIP is one feature of prostate cancer cells. In nude mice, c-FLIP causes androgen-independent growth of prostate cancer cells [28]. In this study, c-FLIP was expressed in prostate cellular tissues. Higher levels of c-FLIP expression were associated with higher GSS and more advanced pathologic stage.
In both HSP27 and c-FLIP, higher expression scores were associated with higher GSS and more advanced pathologic stage. However, expression of the two proteins was not uniform in the same cell lines. Compared to c-FLIP, HSP27 had a higher correlation with pathologic stage and GSS. Patients who could not undergo radical prostatectomy, including those with hormone-refractory prostate cancer, had higher HSP27 and c-FLIP expression because these patients underwent transurethral prostatectomy to alleviate urinary symptoms. These results predict that this group had a higher GSS and more advanced pathologic stage than other cases.
In addition to HSP27 and c-FLIP, there are many proteins related to prostate cancer, including Ki-67, p53, AR, matrix metalloproteinase (MMP)-2, MMP-9, vascular endothelial growth factor, Aurora-A, Bcl-2, clusterin, HSP70, and HSP90 [29]. Studies have investigated Src tyrosine kinase and its relationship to hormone-refractory prostate cancer progression and bone metastasis, as well as signal transducer and activator of transcription 5A kinase and its relationship to hormone-refractory progression. Other factors have also been studied and reported [2730]. In the present study, two factors were selected as the most appropriate factors, considering our focus on the correlation between GSS, pathologic stage, and expressed proteins. Unlike other studies, this study investigated two factors at the same time to evaluate the correlations of HSP27 and c-FLIP expression to GSS and pathologic stage in prostate cancer. Additionally, this study investigated the correlation between the expression levels of the two proteins.
However, there were limitations in verifying the correlation of HSP27 and c-FLIP expression to pathologic factors due to the small number of subjects. Moreover, we could not verify the direct relationship between the MKK series and prostate cancer activity.
And additional limitation was that there were no follow-up results including BCR. Further study is needed to have follow up and results and define the correlation of previously reported factors and their associations with GSS and pathologic stage.
Therefore, an additional large-scale study is necessary to investigate the correlation between HSP27, c-FLIP, and prostate cancer. Large-scale and comparative studies on other factors would also be useful.
Higher GSS and more advanced pathologic stage were associated with higher levels of HSP27 and c-FLIP expression. The expression of HSP27 and c-FLIP was correlated to GSS and pathologic stage in prostate cancer. HSP27 and c-FLIP expression were shown significant related with Gleason sum and pathologic stage at the same time. We recommend a future study about the role of HSP27 and c-FLIP in the initiation, development, and immune control of hormone-refractory prostate cancer.
Figures and Tables
Fig. 1
HSP27expression in prostate tissues (immunohistochemical stain, ×100). (A) HSP27 negative, 0; (B) HSP27<5%, +1; (C) HSP27 5%-50%, +2; (D) HSP27>50%, +3. HSP, heat shock protein.

Fig. 2
c-FLIP expression in prostate tissues (immunohistochemical stain, ×100). (A) c-FLIP negative, 0; (B) c-FLIP reaction<5%, +1; (C) c-FLIP 5%-50%, +2; (D) c-FLIP>50%, +3. c-FLIP, cellular-FLICE inhibitory protein.

Table 1
Patient characteristics in the prostate cancer cell group (n=83)
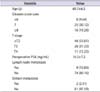
Table 2
HSP27 and c-FLIP expression patterns according to Gleason score sum and pathologic stage

Table 3
Averages of HSP27 andc-FLIP expression according to Gleason score and pathologic stage (n=83)

Table 4
Logistic regression analysis of expression HSP27

Table 5
Logistic regression analysis of expression c-FLIP

Table 6
Association between HSP27, c-FLIP expression and Gleason score sum, pathologic stage

Table 7
Logistic regression analysis of expression HSP 27 and c-FLIP

References
1. Rocchi P, Beraldi E, Ettinger S, Fazli L, Vessella RL, Nelson C, et al. Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis. Cancer Res. 2005; 65:11083–11093.
2. Atkins D, Lichtenfels R, Seliger B. Heat shock proteins in renal cell carcinomas. Contrib Nephrol. 2005; 148:35–56.
3. Rocchi P, So A, Kojima S, Signaevsky M, Beraldi E, Fazli L, et al. Heat shock protein 27 increases after androgen ablation and plays a cytoprotective role in hormone-refractory prostate cancer. Cancer Res. 2004; 64:6595–6602.
4. Hyer ML, Sudarshan S, Kim Y, Reed JC, Dong JY, Schwartz DA, et al. Downregulation of c-FLIP sensitizes DU145 prostate cancer cells to Fas-mediated apoptosis. Cancer Biol Ther. 2002; 1:401–406.
5. Korkolopoulou P, Goudopoulou A, Voutsinas G, Thomas-Tsagli E, Kapralos P, Patsouris E, et al. c-FLIP expression in bladder urothelial carcinomas: its role in resistance to Fasmediated apoptosis and clinicopathologic correlations. Urology. 2004; 63:1198–1204.
6. Lee SH, Kim HS, Kim SY, Lee YS, Park WS, Kim SH, et al. Increased expression of FLIP, an inhibitor of Fas-mediated apoptosis, in stomach cancer. APMIS. 2003; 111:309–314.
7. Kaulfuss S, Burfeind P, Gaedcke J, Scharf JG. Dual silencing of insulin-like growth factor-I receptor and epidermal growth factor receptor in colorectal cancer cells is associated with decreased proliferation and enhanced apoptosis. Mol Cancer Ther. 2009; 8:821–833.
8. Day TW, Sinn AL, Huang S, Pollok KE, Sandusky GE, Safa AR. c-FLIP gene silencing eliminates tumor cells in breast cancer xenografts without affecting stromal cells. Anticancer Res. 2009; 29:3883–3886.
9. Lanneau D, de Thonel A, Maurel S, Didelot C, Garrido C. Apoptosis versus cell differentiation: role of heat shock proteins HSP90, HSP70 and HSP27. Prion. 2007; 1:53–60.
10. Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, et al. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000; 60:7099–7105.
11. Lee SW, Kim EK, Kim SS, Uh HS, Cha KS, Yoo TK. Expression of Heat Shock Protein 27 according to Gleason Score and Pathologic Stage of Prostate Cancer. Korean J Urol. 2009; 50:547–552.
12. Kim SS, Cho HJ, Kang JY, Kang HK, Yoo TK. Inhibition of androgen receptor expression with small interfering RNA enhances cancer cell apoptosis by suppressing survival factors in androgen insensitive, late stage LNCaP cells. ScientificWorldJournal. 2013; 2013:519397.
13. Nakajima A, Komazawa-Sakon S, Takekawa M, Sasazuki T, Yeh WC, Yagita H, et al. An antiapoptotic protein, c-FLIPL, directly binds to MKK7 and inhibits the JNK pathway. EMBO J. 2006; 25:5549–5559.
14. Xu L, Chen S, Bergan RC. MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene. 2006; 25:2987–2998.
15. Lotan TL, Lyon M, Huo D, Taxy JB, Brendler C, Foster BA, et al. Up-regulation of MKK4, MKK6 and MKK7 during prostate cancer progression: an important role for SAPK signalling in prostatic neoplasia. J Pathol. 2007; 212:386–394.
16. Pavese JM, Ogden IM, Voll EA, Huang X, Xu L, Jovanovic B, et al. Mitogen-activated protein kinase kinase 4 (MAP2K4) promotes human prostate cancer metastasis. PLoS One. 2014; 9:e102289.
17. Charafe-Jauffret E, Tarpin C, Bardou VJ, Bertucci F, Ginestier C, Braud AC, et al. Immunophenotypic analysis of inflammatory breast cancers: identification of an 'inflammatory signature'. J Pathol. 2004; 202:265–273.
18. Sarto C, Valsecchi C, Magni F, Tremolada L, Arizzi C, Cordani N, et al. Expression of heat shock protein 27 in human renal cell carcinoma. Proteomics. 2004; 4:2252–2260.
19. Concannon CG, Orrenius S, Samali A. Hsp27 inhibits cytochrome c-mediated caspase activation by sequestering both pro-caspase-3 and cytochrome c. Gene Expr. 2001; 9:195–201.
20. Erkizan O, Kirkali G, Yorukoglu K, Kirkali Z. Significance of heat shock protein-27 expression in patients with renal cell carcinoma. Urology. 2004; 64:474–478.
21. Miyake H, Muramaki M, Kurahashi T, Takenaka A, Fujisawa M. Expression of potential molecular markers in prostate cancer: correlation with clinicopathological outcomes in patients undergoing radical prostatectomy. Urol Oncol. 2010; 28:145–151.
22. Teimourian S, Jalal R, Sohrabpour M, Goliaei B. Down-regulation of Hsp27 radiosensitizes human prostate cancer cells. Int J Urol. 2006; 13:1221–1225.
23. Day TW, Huang S, Safa AR. c-FLIP knockdown induces ligand-independent DR5-, FADD-, caspase-8-, and caspase-9-dependent apoptosis in breast cancer cells. Biochem Pharmacol. 2008; 76:1694–1704.
24. Longley DB, Wilson TR, McEwan M, Allen WL, McDermott U, Galligan L, et al. c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene. 2006; 25:838–848.
25. Zhang X, Jin TG, Yang H, DeWolf WC, Khosravi-Far R, Olumi AF. Persistent c-FLIP(L) expression is necessary and sufficient to maintain resistance to tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in prostate cancer. Cancer Res. 2004; 64:7086–7091.
26. Ye H, Li Y, Melamed J, Pearce P, Wei J, Chiriboga L, et al. Stromal anti-apoptotic androgen receptor target gene c-FLIP in prostate cancer. J Urol. 2009; 181:872–877.
27. Wilson C, Wilson T, Johnston PG, Longley DB, Waugh DJ. Interleukin-8 signaling attenuates TRAIL- and chemotherapy-induced apoptosis through transcriptional regulation of c-FLIP in prostate cancer cells. Mol Cancer Ther. 2008; 7:2649–2661.
28. Gao S, Lee P, Wang H, Gerald W, Adler M, Zhang L, et al. The androgen receptor directly targets the cellular Fas/FasL-associated death domain protein-like inhibitory protein gene to promote the androgen-independent growth of prostate cancer cells. Mol Endocrinol. 2005; 19:1792–1802.
29. Kao CJ, Martiniez A, Shi XB, Yang J, Evans CP, Dobi A, et al. miR-30 as a tumor suppressor connects EGF/Src signal to ERG and EMT. Oncogene. 2014; 33:2495–2503.
30. Roe K, Bratland A, Vlatkovic L, Ragnum HB, Saelen MG, Olsen DR, et al. Hypoxic tumor kinase signaling mediated by STAT5A in development of castration-resistant prostate cancer. PLoS One. 2013; 8:e63723.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download