Abstract
Recently, imaging of prostate cancer has greatly advanced since the introduction of multiparametric magnetic resonance imaging (mpMRI). mpMRI consists of T2-weighted sequences combined with several functional sequences including diffusion-weighted imaging, dynamic contrast-enhanced imaging, and/or magnetic resonance spectroscopy imaging. Interest has been growing in mpMRI because no single MRI sequence adequately detects and characterizes prostate cancer. During the last decade, the role of mpMRI has been expanded in prostate cancer detection, staging, and targeting or guiding prostate biopsy. Recently, mpMRI has been used to assess prostate cancer aggressiveness and to identify anteriorly located tumors before and during active surveillance. Moreover, recent studies have reported that mpMRI is a reliable imaging modality for detecting local recurrence after radical prostatectomy or external beam radiation therapy. In this regard, some urologic clinical practice guidelines recommended the use of mpMRI in the diagnosis and management of prostate cancer. Because mpMRI is the evolving reference standard imaging modality for prostate cancer, urologists should acquire cutting-edge knowledge about mpMRI. In this article, we review the literature on the use of mpMRI in urologic practice and provide a brief description of techniques. More specifically, we state the role of mpMRI in prostate biopsy, active surveillance, high-risk prostate cancer, and detection of recurrence after radical prostatectomy.
Recently, interest has been growing in prostate magnetic resonance imaging (MRI) because of improvements in the accuracy of prostate cancer detection and characterization via the combination of conventional T1-weighted imaging and T2-weighted imaging (T2WI) with various functional imaging modalities [1]. These functional imaging modalities include diffusion-weighted MRI (DWI), dynamic contrast-enhanced MRI (DCEI), and magnetic resonance spectroscopy imaging (MRSI). Combined conventional MRI with DWI, DCEI, and/or MRSI is known as multiparametric MRI (mpMRI) [2].
mpMRI is currently regarded as the most sensitive and specific imaging technique for prostate cancer evaluation, including detection, staging, localization, and aggressiveness measurement [34]. mpMRI can also help to detect tumors missed on conventional biopsy and allow for targeted biopsies [56]. Furthermore, mpMRI has improved many aspects of prostate cancer management. mpMRI is used to monitor disease progression during active surveillance and recurrence after definite treatment [78]. Although mpMRI is not routinely recommended, recent guidelines have suggested that mpMRI can help in deciding on enrolment for active surveillance, in identifying anteriorly located prostate cancer in patients with suspicious negative biopsy results, and in deciding on nerve-sparing procedures in intermediate and high-risk disease [9101112].
This article presents an overview of mpMRI and the individual mpMRI techniques with their strong and weak points for clinical application. We also discuss the emerging role of MRI in detecting and managing prostate cancer.
Currently, mpMRI is regarded as the reference standard imaging modality for prostate cancer because a single MRI sequence cannot adequately detect and characterize prostate cancer. Although the ideal set of sequences for prostate mpMRI has not been determined, mpMRI is composed of high-resolution T2WI, DWI, and DCEI with optional MRSI. The principle, strong and weak points of each mpMRI sequence are summarized in Table 1. In addition, an image of a radical prostatectomy specimen and the corresponding mpMRI images are presented in Fig. 1 to aid in understanding the characteristics of each mpMRI sequence.
T2WI, which reflects the water content of tissue, is the basis of mpMRI. Because of the high resolution and sharp demarcation of the prostate capsule, T2WI can be used to determine prostate zonal anatomy and prostate cancer staging. In contrast to cancer in other organs, prostate cancer presents low signal intensity compared with adjacent glandular tissue because the abundant amount of water in the normal gland demonstrates high signal intensity on T2WI. This signal difference between normal and cancer tissue helps in cancer detection in the gland-rich peripheral zone. However, cancer identification on T2WI may be limited in the transitional zone, which does not contain a large amount of water. Moreover, the T2 shortening effect by biopsy-induced hemorrhage decreases signal intensity even in noncancerous tissue. Therefore, despite satisfactory performance as reported by early studies, recent literature has demonstrated the limitations of T2WI for prostate cancer detection. Therefore, the sensitivity and specificity of T2WI show significant variation in studies, i.e., 55%-88% for sensitivity and 67%-82% for specificity [13]. Furthermore, such potential drawbacks of T2WI have introduced the need for mpMRI.
DWI, which quantifies the degree of water diffusing in the tissue, can be applied to prostate cancer detection because the high cellularity and abundant intra- and intercellular membranes in cancer tissue generally result in diffusion restriction. On an apparent diffusion coefficient (ADC) map, prostate cancer shows a lower signal intensity than do adjacent normal glands. Investigators have validated the usefulness of the ADC map for cancer detection, which has mostly demonstrated addition of the ADC map to T2WI to improve cancer detection. Moreover, assessment of tumor aggressiveness using DWI is attracting much attention, although accurate Gleason score prediction is difficult [14]. Although DWI can increase the sensitivity of prostate cancer detection, it is also limited for visualizing cancer tissue in the transitional zone because BPH-related nodules also demonstrate decreased diffusion. Moreover, tumor necrosis may manifest increased water diffusion, i.e., high signal intensity on an ADC map. More vulnerability to artifacts, relatively low signal-to-noise ratio, and low spatial resolution are also potential drawbacks of DWI. As a result, the sensitivity (65%-84%) and specificity (77%-87%) of the combined approach of DWI and T2WI has shown significant variability in different studies [13].
DCEI is an imaging modality that is designed to evaluate the status of tumor angiogenesis. DCEI requires the acquisition of repeat gradient echo images before and after injection of contrast materials such as chelated gadolinium. Owing to rapid imaging, DCEI provides the time-intensity curve in each voxel. Because tumors are evidently associated with neoangiogenesis that induces an increase in the blood volume and transvascular permeability, tracing the dynamic flux of the contrast agent with DCEI shows strong and rapid contrast enhancement. Therefore, DCEI helps to monitor treatment effects as well as cancer detection. Recently, studies have also reported that DCEI can improve diagnostic performance for detecting local recurrence in patients who undergo radical prostatectomy [15]. However, DCEI may cause false-positive diagnosis because inflammation is also accompanied by increased vascularity. Patient motion and peristalsis of the rectum during imaging may cause misregistration in imaging series, thereby disturbing the analysis of the time-intensity curve. The reported sensitivity and specificity of DCEI alone for prostate cancer detection also varies by reports (46%-90% for sensitivity and 74%-96% for specificity) [15].
MRSI depicts the relative concentration of certain metabolites. MRSI distinguishes prostate cancer with normal prostate tissue on the basis of the choline plus creatinine-to-citrate ratio. Increased choline and creatinine are observed in areas of proliferative prostate cancer. Citrate is produced by the normal prostate epithelium and is decreased in the area of prostate cancer. Thus, a higher choline plus creatinine-to-citrate ratio is observed in the areas of high tumor concentration. MRSI also provides information about the aggressiveness of prostate cancer. The sensitivity and specificity of MRSI alone are from 75% to 89% and from 77% to 91%, respectively [16]. MRSI has some limitations because the method takes additional time and requires more expertise than do other functional sequences.
The recommendation for mpMRI varies in different clinical practice guidelines. However, recently published guidelines from the European Association of Urology (EAU), the American Urological Association (AUA), and the National Comprehensive Cancer Network (NCCN) mention the potential role of mpMRI in prostate biopsy, active surveillance, and recurrent prostate cancer. Moreover, the EAU guideline recommended mpMRI in several fields of prostate cancer management.
For prostate cancer diagnosis, the EAU guideline suggested that mpMRI can be used to trigger a targeted repeat biopsy [9]. The EAU guideline suggested that mpMRI followed by transrectal ultrasonography (TRUS)-guided or MRI-guided biopsies might be particularly useful at identifying prostate cancer located in anterior regions, which is usually missed by conventional TRUS-guided biopsy. However, the EAU concluded that these results need to be verified and that the cost-effectiveness of mpMRI before confirmatory biopsy is questionable. In patients with low-risk prostate cancer, mpMRI is not recommended for staging purposes. The EAU suggested that mpMRI should be used in local staging only if its results change patient management. However, mpMRI can aid in deciding when to perform nerve-sparing procedures in intermediate and high-risk disease. In patients with biochemical recurrence who are candidates for salvage therapy, the EAU guideline suggested that mpMRI can guide biopsy [10]. However, the EAU guideline mentioned that it remains to be determined whether mpMRI will be able to correctly detect post-prostatectomy local recurrences in patients with a prostate-specific antigen (PSA) level <0.5 ng/mL. When mpMRI is applied, a standardized scoring system, such as the Magnetic Resonance Prostate Imaging Reporting and Data System (MR PI-RADS) or Standards of Reporting for MRI-Targeted Biopsy Studies (START), is highly recommended.
The NCCN guideline suggested that mpMRI may be considered for patients who choose active surveillance to exclude the presence of anterior cancer if the PSA rises and systematic prostate biopsy results remain negative [11]. mpMRI may provide additional information in certain clinical settings, such as rising PSA or a positive digital rectal exam result after radiotherapy in the setting of a negative prostate biopsy. mpMRI may be particularly useful in men being considered for local salvage therapy. However, they concluded that mpMRI is not recommended for routine use.
The AUA/ASTRO (American Society for Radiation Oncology) guidelines also suggested that mpMRI could identify sites of local recurrence and improve salvage radiation targeting [12]. Moreover, mpMRI could evaluate the need to add androgen deprivation in patients with massive recurrences not appropriate for conventional radiotherapy.
Although prostate mpMRI has been widely used, interpretation of mpMRI has not been standardized until quite recently. Before the introduction of the PI-RADS classification, a Likert scale of scores from 1 to 5 was used to characterize a radiologist's level of suspicion for focal lesions based on impression without fixed criteria. Lack of a standardized mpMRI reporting system led to substantial variability in interpretation.
In 2012, the European Society of Urogenital Radiology (ESUR) introduced the PI-RADS classification for structured reporting of mpMRI [2]. The PI-RADS classification is the first and most widely accepted mpMRI scoring system. In PI-RADS, each suspicious lesion is scored on a 5-point scale for each sequence including T2WI, DWI, DCEI, and MRSI, with 1 being benign and 5 being highly suspicious of malignancy. The maximum score depends on the number of sequences performed. In addition, the scoring system recommends that a diagnosis of suspected prostate cancer be made if the PI-RADS score is 4 or higher (if 3 sequences were performed, sum of score ≥10, if 4 sequences were preformed, sum of score ≥13). Recent meta-analysis reported that PI-RADS appeared to have appropriate diagnostic accuracy with pooled sensitivity of 78% and specificity of 79% [17]. However, those authors suggested that the ESUR guideline should be improved in two aspects: the provision of clear instructions for overall score calculation (sum of score vs. overall 5-point scale) and recommendations regarding the use of a threshold for prostate biopsy. Because of heterogeneity among studies, a cutoff score for prostate biopsy cannot be provided.
Recently, PI-RADS version 2 dealt with these problems [18]. PI-RADS version 2 assessed the probability of clinically significant prostate cancer by using a 5-point scale based on T2WI, DWI, and DCEI. In PI-RADS version 2, prostate biopsy should be considered for PI-RADS assessment category 4 or 5. For PI-RADS assessment category 2 or 3, biopsy may or may not be appropriate, depending on other clinical variables. In PI-RADS version 2, the primary determining sequence for a lesion located in the peripheral zone is DWI and that for a lesion located in the transition zone is T2WI. The score of the primary determining sequence and PI-RADS assessment category correspond closely regardless of the score of the other sequences. DCEI plays only a minor role in determining PI-RADS assessment category, and each lesion gets a (+) or (-) score based on DCEI.
Another mpMRI scoring system called the National Cancer Institute (NCI) MP-MRI evaluation score also exists, although this system has not been widely used [19]. The NCI MP-MRI scoring system is based on the number of positive sequences for prostate cancer. In this system, lesions are separated into 3 categories of low, moderate, and high risk. A lesion is considered low risk if positive on 1 or 2 of the 3 sequences. If all 3 sequences are positive, the lesion is considered moderate or high risk. A lesion is considered high risk if all 4 parameters are positive, including MRSI.
An international working group introduced standards of reporting MRI-targeted biopsy studies of the prostate, also known as START [20]. They suggested the START checklist, which contain the panel's recommendations of statements to include in a report of MRI-targeted biopsy. According to this checklist, the field strength of the magnet, the specific coils being used, a brief description of the sequences, the reporting method used, and the experience of the reporting radiologist should be reported.
TRUS-guided 12-core biopsy is the generally recommended method for prostate biopsy and has been validated in enhanced cancer detection. However, about 20% of patients are reported to have prostate cancer on a repeat biopsy despite a previous negative biopsy result for cancer [21]. Moreover, a considerable number of patients experience an upgrade in postprostatectomy Gleason score [22]. This diagnostic uncertainty may lead patients to repeat prostate biopsy, delayed treatment, or overtreatment.
The use of mpMRI to improve the accuracy of prostate biopsy has increased because of several potential benefits of MRI-targeted or guided biopsy. One benefit is the precise localization of significant prostate cancer before biopsy, which may result in accurate pretreatment risk stratification. Recent studies have reported that localization of the index lesion by use of mpMRI is reliable [2324]. In this regard, mpMRI is reported as an effective method for detecting and localizing clinically significant prostate cancer, especially in men with negative biopsy results [25]. Moreover, mpMRI increases repeat biopsy yields by identifying anteriorly located prostate cancer [26272829].
Several prostate biopsy methods are available depending on the means of applying MRI. The first method is visual estimation MRI-targeted TRUS-guided biopsy. This method carries a learning curve and lacks real-time feedback, although the additional cost is minimal. However, this method is limited because of inconsistencies in targeting precision. Another method is software coregistered MRI/TRUS fusion biopsy, which overcomes the limitation of the previous method. MRI/TRUS fusion biopsy has greater reproducibility because of less operator dependence and real-time feedback. However, the additional cost of the device and software may be another problem. In-bore MR-guided biopsy also has real-time feedback of biopsy needle placement in addition to increased accuracy. However, application of this method is limited because of economic feasibility and inability to routinely sample the remaining prostate. Recently, prostate biopsy using an MRI-compatible robot was introduced and reported as a feasible method [3031]. The disadvantages of manually performed MRI-guided prostate biopsy might be eliminated with robotic biopsy.
Recent studies that have reported the accuracy of mpMRI targeted or guided biopsy are summarized in Table 2. MRI-targeted biopsy was reported to improve the detection rate of prostate cancer compared with conventional biopsy when a similar number of cores is biopsied [323334]. Moreover, most studies reported that MRI-targeted or guided biopsy yielded a comparable prostate cancer detection rate with a decreased number of cores compared with conventional 12-core biopsy [3536373839404142]. Although two studies reported a decreased detection rate, significant prostate cancer was comparably detected despite the lower number of biopsied cores [4344]. The prostate cancer detection rate with the use of MRI targeted or guided 1- to 5-core biopsy as a first-round prostate biopsy method was comparable with that of 12-core TRUS-guided biopsy, although MRI-targeted biopsy showed slightly inferior results compared with MRI/TRUS fusion or MRI-guided biopsy [37414243]. Moreover, in men with negative biopsy results, the number of cores biopsied could be reduced with mpMRI instead of the standard 12-core biopsy [39]. In most studies, mpMRI consisted of DWI and DCEI, although we cannot determine the most appropriate set of sequences for prostate biopsy. The combination of DWI and DCEI showed a prostate cancer detection rate similar to that of mpMRI consisting of DWI, DCEI, and MRSI [364345].
Active surveillance is regarded as a standard treatment method for patients with low-risk and very-low-risk prostate cancer, which has indolent characteristics. Patients who undergo active surveillance are followed up with a regular PSA level test, digital rectal examination, and repeat prostate biopsy. Current practice guidelines do not recommend mpMRI for prostate cancer patients eligible for active surveillance. However, mpMRI has an emerging potential role in detecting clinically significant prostate cancer before enrollment in active surveillance and in reducing the number of repeat biopsies during active surveillance follow-up.
One of the most important aspects of active surveillance is selection of appropriate patients. In this regard, risk misclassification can be a huge problem. Some recent studies have suggested the promising detection rate of clinically significant prostate cancer in an active surveillance cohort (Table 3). However, the detection rate of significant prostate cancer varied widely within the studies if the definition of significant prostate cancer was changed [19464748]. Nevertheless, at least a quarter of active surveillance candidates with a suspicious lesion on mpMRI are reclassified as having clinically significant prostate cancer. Moreover, more than one-half of patients are identified as patients with significant prostate cancer if studies using prostatectomy specimen pathology as a standard reference are selected [47495051]. In other words, significant prostate cancer could be more precisely excluded before active surveillance enrollment if a lesion is not seen on mpMRI.
mpMRI during active surveillance was recently reported as a promising follow-up modality, although more studies on these fields should be done to gain a better understanding. Among functional sequences, DWI was reported as a potential monitoring sequence for patients with early prostate cancer who opt for active surveillance [52]. In that study, there was a significant decrease in ADC values over time in patients who progressed to radical treatment, although ADC values were stable in patients who did not progress to radical treatment. Moreover, Siddiqui et al. suggested that the active surveillance nomogram using mpMRI might reasonably decrease the number of repeat biopsies in patients undergoing active surveillance by as much as 68%, which agrees with other articles [535455].
High-risk prostate cancer has been defined differently in several guidelines and articles. However, the most generally accepted definition of high-risk prostate cancer is a clinical T stage≥T2c or T3a in addition to Gleason score≥8 or a PSA level>20 ng/mL [1156]. Recently, some articles have reported mpMRI as a useful modality for predicting pathological outcomes in patients with high-risk prostate cancer (Table 4).
mpMRI has shown high specificity for detecting extracapsular extension (ECE), although sensitivity varied according to the study [57585960]. Additional mpMRI combined with conventional clinical variables increased prediction accuracy for recurrence after prostatectomy in high-risk disease [57]. Moreover, mpMRI improved decision-making to preserve the neurovascular bundle [60]. However, the negative predictive value for predicting ECE, which is important for deciding on nerve-sparing radical prostatectomy, was not sufficiently high in other studies [575859]. Moreover, mpMRI was limited because it was unable to identify and localize focal ECE, which is a large majority of ECE [61]. Seminal vesicle invasion could be also predicted by using mpMRI with a positive predictive value of 62% to 95% and a negative predictive value of 73% to 83% [5759].
The sensitivity and specificity of mpMRI for detecting lymph node invasion was 14% to 33% and 91% to 97%, respectively [5759]. Because of low sensitivity, mpMRI was not routinely indicated for lymph node workup in patients without suspicious lymph nodes on computed tomography (CT) [62]. Moreover, positron emission tomography-CT showed higher sensitivity and specificity for detecting lymph node invasion compared with mpMRI [59]. Despite these limitations, whole-body mpMRI as a one-stop imaging modality was recently proposed instead of prostate mpMRI plus bone scan plus CT in high-risk patients [63]. Although whole-body mpMRI was reported to be superior to a combination of imaging modalities, these results should be verified.
Most postprostatectomy recurrent prostate cancer is diagnosed by PSA elevation. Once PSA increment is detected, it is essential to identify whether prostate cancer has recurred at a local or a distant site to determine the treatment modalities. In current practice, imaging or pathological evidence of local recurrence is not necessary to initiate local salvage treatment because current imaging techniques cannot adequately detect small-sized local recurrence.
In a recent meta-analysis, mpMRI was reported to have sufficient accuracy for detecting local recurrence in patients with low PSA and small-sized recurrence [64]. Recently, an increasing number of studies have been published reporting the acceptable diagnostic accuracy of mpMRI for detecting local recurrence (Table 5). Among the functional sequences, DCEI has been regarded as the most reliable sequence in detecting local recurrence after prostatectomy [6566]. However, it must be taken into account that vascularity and contrast enhancement can be reduced in patients who have received androgen deprivation therapy. In this regard, the accuracy of DCEI might be reduced in patients who undergo androgen deprivation therapy. Sensitivity and specificity of DCEI alone for detecting local recurrence after radical prostatectomy range from 88% to 100% and from 45% to 97%, respectively [65666768]. Moreover, DCEI increased interobserver agreement and addition of DCEI to T2WI significantly increased accuracy for detecting local recurrence [67].
Recently, some studies have shown that DWI is also a reliable sequence in detecting local recurrence [6869]. Moreover, the combination of DWI and DCEI seemed to have more consistent specificity of 82% to 87% compared with DCEI alone [666970]. Accuracy of combined functional sequences has not been sufficiently reported. According to a recent study, T2WI plus DCEI has the highest sensitivity of 97% followed by DCEI alone and T2WI plus DWI plus DCEI [66].
mpMRI, which is composed of T2WI and several functional sequences, is regarded as the single most accurate imaging modality for characterizing prostate cancer. Recently, the role of mpMRI has been expanded to prostate biopsy, active surveillance, advanced disease detection, and local recurrence detection after radical prostatectomy. In this regard, urologists should acquire cutting-edge knowledge about mpMRI because it is a rapidly evolving imaging modality.
Figures and Tables
Fig. 1
A 72-year-old patient with prostate cancer. (A) On a picture of radical prostatectomy specimen, arrows indicate prostate cancer. (B) On T2-weighted imaging, prostate cancer shows slightly low signal intensity although the contrast between prostate cancer and adjacent normal tissue is not apparent. (C) On color-coded map of apparent diffusion coefficient, prostate cancer shows significantly decreased values, presented as dark blue color. (D) On initial area under the curve map derived from dynamic contrast-enhanced magnetic resonance imaging, prostate cancer shows increased vascularity, presented as yellow and green color.
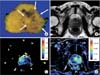
Table 1
Principles and characteristics of T2WI and each functional sequence

Table 2
Recent articles reporting the role of mpMRI in prostate biopsy

| Source | Year | Patients No. | Previous biopsy | Sequence | Navigation | Biopsy core (n) | Prostate cancer detection rate | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | MRI | MRI | TRUS | Overall | Significant cancer | |||||||
| MRI | TRUS | MRI | TRUS | |||||||||
| Abdi et al. [32] | 2015 | 192 | 86 | (-) Results | DWI, DCEI | MRI-targeted | 14 | 12 | 42% | 19% | 35% | 14% |
| Kim et al. [33] | 2015 | 68 | 34 | Not applicable | DWI, DCEI | MRI-targeted | 12 | 12 | 54% | 21% | 25% | 7% |
| Jambor et al. [43] | 2014 | 55 | 55 | No previous biopsy | DWI, DCEI, MRSI | MRI-targeted | 1-5 | 12 | 63% | 91% | 60% | 74% |
| Puech et al. [35] | 2013 | 95 | 95 | Not applicable | DWI, DCEI | MRI-targeted | 4 | 12 | 69% | 59% | 67% | 52% |
| Javali et al. [34] | 2014 | 278 | 140 | Not applicable | MRSI | MRI-targeted | 6 or 12 | 6 or 12 | 24% | 10% | NA | NA |
| Siddiqui et al. [36] | 2015 | 1,003 | 1,003 | Not applicable | DWI, DCEI, MRSI | MR/TRUS fusion | 5 | 12 | 46% | 47% | 17% | 12% |
| Brock et al. [44] | 2014 | 121 | 121 | (-) Results | DWI, DCEI | MR/TRUS fusion | 4 | 12 | 26% | 38% | 24% | 28% |
| Mozer et al. [37] | 2015 | 152 | 152 | No previous biopsy | DWI, DCEI | MR/TRUS fusion | 2-3 | 12 | 54% | 57% | 43% | 37% |
| Rastinehad et al. [38] | 2014 | 105 | 105 | Not applicable | DWI, DCEI | MR/TRUS fusion | 4 | 12 | 51% | 49% | 45% | 32% |
| Walton Diaz et al. [45] | 2013 | 649 | 649 | Not applicable | DWI, DCEI, MRSI | MR/TRUS fusion | 2/lesion | 12 | 55% | NA | NA | NA |
| Sonn et al. [39] | 2014 | 105 | 105 | (-) Results | DWI, DCEI | MR/TRUS fusion | 4 | 12 | 34% | 27% | 22% | 15% |
| Ukimura et al. [40] | 2015 | 127 | 127 | Not applicable | DWI, DCEI | MR/TRUS fusion | 3 | 11 | 61% | 41% | 43% | 23% |
| Quentin et al. [41] | 2014 | 128 | 128 | No previous biopsy | DWI, DCEI | MRI-guided | 2/lesion | 12 | 53% | 53% | 45% | 42% |
| Pokorny et al. [42] | 2014 | 223 | 223 | No previous biopsy | DWI, DCEI | MRI-guided | 3 | 12 | 70% | 57% | 67% | 35% |
Table 3
Recent studies of the role of mpMRI in determining active surveillance eligibility

| Source | Year | Patients No. | Sequence | Standard reference | Definition of sPCa | sPCa detection rate |
|---|---|---|---|---|---|---|
| Kim et al. [49] | 2015 | 287 | DWI | Prostatectomy specimen | Stage≥pT3 or TV≥0.5 mL or GS pattern ≥4 | 75% |
| Abd-Alazeez et al. [46] | 2014 | 194 | DWI, DCEI | Biopsy | Multiple definitions | 25%-41% |
| Park et al. [50] | 2014 | 298 | DWI, DCEI | Prostatectomy specimen | Pathologic stage ≥ T3 or GS ≥ 7 | 52% |
| Stamatakis et al. [19] | 2013 | 85 | DWI, DCEI, MRSI | Biopsy | Multiple definitions | 38%-56% |
| Turkbey et al. [51] | 2013 | 133 | DWI, DCEI, MRSI | Prostatectomy specimen | Stage≥pT3 or TV≥0.5 mL or GS pattern ≥4 | 99% |
| Shukla-Dave et al. [47] | 2012 | 181 | MRSI | Prostatectomy specimen | Multiple definitions | 66%-77% |
| Thompson et al. [48] | 2014 | 150 | DWI, DCEI, MRSI | Biopsy or prostatectomy specimen | Multiple definitions | 30%-41% |
Table 4
Recent studies of the role of mpMRI in high-risk prostate cancer

| Source | Year | Patients No. | Sequence | Definition of high-risk prostate cancer | Factor | Sen (%) | Spe (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|---|
| Jeong et al. [57] | 2013 | 922 | DWI | PSA≥20 ng/mL, GS≥8, clinical stage≥T2c | ECE | 43 | 84 | 79 | 52 |
| SVI | 35 | 94 | 62 | 83 | |||||
| LNI | 14 | 97 | 23 | 95 | |||||
| Pinaquy et al. [59] | 2015 | 47 | DWI | PSA≥20 ng/mL, GS≥8, clinical stage≥T2c | ECE | 72 | 77 | 86 | 59 |
| SVI | 73 | 95 | 95 | 73 | |||||
| LNI | 33 | 91 | 50 | 84 | |||||
| Somford et al. [58] | 2013 | 183 | DWI, DCEI | PSA≥20 ng/mL, GS≥8, clinical stage≥T2c | ECE | 65 | 73 | 89 | 38 |
| Park et al. [60] | 2014 | 67 | DWI, DCEI | PSA≥20 ng/mL, GS≥8, clinical stage≥T2c | ECE | 80 | 85 | 89 | 74 |
mpMRI, multiparametric magnetic resonance imaging; Sen, sensitivity; Spe, specificity; PPV, positive predictive value; NPV, negative predictive value; DWI, diffusion-weighted imaging; PSA, prostate-specific antigen; GS, Gleason score; ECE; extracapsular extension; SVI, seminal vesicle invasion; LNI, lymph node invasion; DCEI, dynamic contrast-enhanced imaging.
Table 5
Recent studies of the role of mpMRI in detecting local recurrence after radical prostatectomy

| Source | Year | Patients No. | Sequence | Standard reference | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Wassberg et al. [67] | 2012 | 52 | DCEI | Biopsy | Reader 1: 88% | Reader 1: 74% |
| Reader 2: 100% | Reader 2: 58% | |||||
| Panebianco et al. [68] | 2013 | 242 | DWI, DCEI | Biopsy, PSA reduction after RT | DCEI: 98%-100% | DCEI: 94%-97% |
| DWI: 97%-98% | DWI: 95%-96% | |||||
| Roy et al. [66] | 2013 | 28 | DWI, DCEI, MRSI | Biopsy | DCEI: 97% | |
| DWI: 65% | ||||||
| MRSI: 53% | ||||||
| DWI+DCEI: 94% | ||||||
| All sequences: 74% | ||||||
| Kitajima et al. [70] | 2014 | 115 | DWI, DCEI | Biopsy, PSA reduction after RT | 89% | 85% |
| Linder et al. [65] | 2014 | 187 | DCEI | Biopsy, PSA reduction after RT, increased size on imaging study | 91% | 45% |
| Cha et al. [69] | 2014 | 57 | DWI, DCEI | Biopsy | Reader 1: 79% | Reader 1: 87% |
| Reader 2: 90% | Reader 2: 82% |
References
1. Hoeks CM, Barentsz JO, Hambrock T, Yakar D, Somford DM, Heijmink SW, et al. Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology. 2011; 261:46–66.
2. Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012; 22:746–757.
3. Johnson LM, Turkbey B, Figg WD, Choyke PL. Multiparametric MRI in prostate cancer management. Nat Rev Clin Oncol. 2014; 11:346–353.
4. Futterer JJ, Briganti A, DeVisschere P, Emberton M, Giannarini G, Kirkham A, et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol. 2015; 02. 2. [Epub]. DOI: 10.1016/j.eururo.2015.01.013.
5. Murphy G, Haider M, Ghai S, Sreeharsha B. The expanding role of MRI in prostate cancer. AJR Am J Roentgenol. 2013; 201:1229–1238.
6. Bjurlin MA, Meng X, Le Nobin J, Wysock JS, Lepor H, Rosenkrantz AB, et al. Optimization of prostate biopsy: the role of magnetic resonance imaging targeted biopsy in detection, localization and risk assessment. J Urol. 2014; 192:648–658.
7. Barchetti F, Panebianco V. Multiparametric MRI for recurrent prostate cancer post radical prostatectomy and postradiation therapy. Biomed Res Int. 2014; 2014:316272.
8. Dianat SS, Carter HB, Macura KJ. Performance of multiparametric magnetic resonance imaging in the evaluation and management of clinically low-risk prostate cancer. Urol Oncol. 2014; 32:39.e1–39.e10.
9. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intentupdate 2013. Eur Urol. 2014; 65:124–137.
10. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014; 65:467–479.
11. Mohler JL, Kantoff PW, Armstrong AJ, Bahnson RR, Cohen M, D'Amico AV, et al. Prostate cancer, version 2.2014. J Natl Compr Canc Netw. 2014; 12:686–718.
12. Thompson IM, Valicenti RK, Albertsen P, Davis BJ, Goldenberg SL, Hahn C, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013; 190:441–449.
13. Wu LM, Xu JR, Ye YQ, Lu Q, Hu JN. The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: a systematic review and meta-analysis. AJR Am J Roentgenol. 2012; 199:103–110.
14. Tamada T, Sone T, Jo Y, Yamamoto A, Ito K. Diffusion-weighted MRI and its role in prostate cancer. NMR Biomed. 2014; 27:25–38.
15. Verma S, Turkbey B, Muradyan N, Rajesh A, Cornud F, Haider MA, et al. Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR Am J Roentgenol. 2012; 198:1277–1288.
16. Kumar V, Jagannathan NR, Thulkar S, Kumar R. Prebiopsy magnetic resonance spectroscopy and imaging in the diagnosis of prostate cancer. Int J Urol. 2012; 19:602–613.
17. Hamoen EH, de Rooij M, Witjes JA, Barentsz JO, Rovers MM. Use of the prostate imaging reporting and data system (PI-RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: a diagnostic meta-analysis. Eur Urol. 2015; 67:1112–1121.
18. Prostate Imaging Reporting and Data System (PI-RADS) [Internet]. Reston (VA): American College of Radiology;cited 2015 Mar 5. Available from: http://www.acr.org/Quality-Safety/Resources/PIRADS/.
19. Stamatakis L, Siddiqui MM, Nix JW, Logan J, Rais-Bahrami S, Walton-Diaz A, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer. 2013; 119:3359–3366.
20. Moore CM, Kasivisvanathan V, Eggener S, Emberton M, Futterer JJ, Gill IS, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol. 2013; 64:544–552.
21. Singh H, Canto EI, Shariat SF, Kadmon D, Miles BJ, Wheeler TM, et al. Predictors of prostate cancer after initial negative systematic 12 core biopsy. J Urol. 2004; 171:1850–1854.
22. Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol. 2012; 61:1019–1024.
23. Rosenkrantz AB, Deng FM, Kim S, Lim RP, Hindman N, Mussi TC, et al. Prostate cancer: multiparametric MRI for index lesion localization: a multiple-reader study. AJR Am J Roentgenol. 2012; 199:830–837.
24. Rud E, Klotz D, Rennesund K, Baco E, Berge V, Lien D, et al. Detection of the index tumour and tumour volume in prostate cancer using T2-weighted and diffusion-weighted magnetic resonance imaging (MRI) alone. BJU Int. 2014; 114(6b):E32–E42.
25. Hambrock T, Somford DM, Hoeks C, Bouwense SA, Huisman H, Yakar D, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol. 2010; 183:520–527.
26. Ahmed HU, Kirkham A, Arya M, Illing R, Freeman A, Allen C, et al. Is it time to consider a role for MRI before prostate biopsy? Nat Rev Clin Oncol. 2009; 6:197–206.
27. Lawrentschuk N, Haider MA, Daljeet N, Evans A, Toi A, Finelli A, et al. 'Prostatic evasive anterior tumours': the role of magnetic resonance imaging. BJU Int. 2010; 105:1231–1236.
28. Volkin D, Turkbey B, Hoang AN, Rais-Bahrami S, Yerram N, Walton-Diaz A, et al. Multiparametric magnetic resonance imaging (MRI) and subsequent MRI/ultrasonography fusion-guided biopsy increase the detection of anteriorly located prostate cancers. BJU Int. 2014; 114(6b):E43–E49.
29. Komai Y, Numao N, Yoshida S, Matsuoka Y, Nakanishi Y, Ishii C, et al. High diagnostic ability of multiparametric magnetic resonance imaging to detect anterior prostate cancer missed by transrectal 12-core biopsy. J Urol. 2013; 190:867–873.
30. Zangos S, Melzer A, Eichler K, Sadighi C, Thalhammer A, Bodelle B, et al. MR-compatible assistance system for biopsy in a high-field-strength system: initial results in patients with suspicious prostate lesions. Radiology. 2011; 259:903–910.
31. Tilak G, Tuncali K, Song SE, Tokuda J, Olubiyi O, Fennessy F, et al. 3T MR-guided in-bore transperineal prostate biopsy: a comparison of robotic and manual needle-guidance templates. J Magn Reson Imaging. 2014; 09. 27. [Epub]. DOI: 10.1002/jmri.24770.
32. Abdi H, Zargar H, Goldenberg SL, Walshe T, Pourmalek F, Eddy C, et al. Multiparametric magnetic resonance imaging-targeted biopsy for the detection of prostate cancer in patients with prior negative biopsy results. Urol Oncol. 2015; 33:165.e1–165.e7.
33. Kim EH, Vemana G, Johnson MH, Vetter JM, Rensing AJ, Strother MC, et al. Magnetic resonance imaging-targeted vs. conventional transrectal ultrasound-guided prostate biopsy: single-institution, matched cohort comparison. Urol Oncol. 2015; 33:109.e1–109.e6.
34. Javali TD, Dwivedi DK, Kumar R, Jagannathan NR, Thulkar S, Dinda AK. Magnetic resonance spectroscopy imaging-directed transrectal ultrasound biopsy increases prostate cancer detection in men with prostate-specific antigen between 4-10 ng/mL and normal digital rectal examination. Int J Urol. 2014; 21:257–262.
35. Puech P, Rouviere O, Renard-Penna R, Villers A, Devos P, Colombel M, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy: prospective multicenter study. Radiology. 2013; 268:461–469.
36. Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015; 313:390–397.
37. Mozer P, Roupret M, Le Cossec C, Granger B, Comperat E, de Gorski A, et al. First round of targeted biopsies using magnetic resonance imaging/ultrasonography fusion compared with conventional transrectal ultrasonography-guided biopsies for the diagnosis of localised prostate cancer. BJU Int. 2015; 115:50–57.
38. Rastinehad AR, Turkbey B, Salami SS, Yaskiv O, George AK, Fakhoury M, et al. Improving detection of clinically significant prostate cancer: magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy. J Urol. 2014; 191:1749–1754.
39. Sonn GA, Chang E, Natarajan S, Margolis DJ, Macairan M, Lieu P, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014; 65:809–815.
40. Ukimura O, Marien A, Palmer S, Villers A, Aron M, de Castro Abreu AL, et al. Trans-rectal ultrasound visibility of prostate lesions identified by magnetic resonance imaging increases accuracy of image-fusion targeted biopsies. World J Urol. 2015; 02. 6. [Epub]. DOI: 10.1007/s00345-015-1501-z.
41. Quentin M, Blondin D, Arsov C, Schimmoller L, Hiester A, Godehardt E, et al. Prospective evaluation of magnetic resonance imaging guided in-bore prostate biopsy versus systematic transrectal ultrasound guided prostate biopsy in biopsy naïve men with elevated prostate specific antigen. J Urol. 2014; 192:1374–1379.
42. Pokorny MR, de Rooij M, Duncan E, Schroder FH, Parkinson R, Barentsz JO, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultra-soundguided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol. 2014; 66:22–29.
43. Jambor I, Kahkonen E, Taimen P, Merisaari H, Saunavaara J, Alanen K, et al. Prebiopsy multiparametric 3T prostate MRI in patients with elevated PSA, normal digital rectal examination, and no previous biopsy. J Magn Reson Imaging. 2015; 41:1394–1404.
44. Brock M, Loppenberg B, Roghmann F, Pelzer A, Dickmann M, Becker W, et al. Impact of real-time elastography on magnetic resonance imaging/ultrasound fusion guided biopsy in patients with prior negative prostate biopsies. J Urol. 2015; 193:1191–1197.
45. Walton Diaz A, Hoang AN, Turkbey B, Hong CW, Truong H, Sterling T, et al. Can magnetic resonance-ultrasound fusion biopsy improve cancer detection in enlarged prostates? J Urol. 2013; 190:2020–2025.
46. Abd-Alazeez M, Ahmed HU, Arya M, Allen C, Dikaios N, Freeman A, et al. Can multiparametric magnetic resonance imaging predict upgrading of transrectal ultrasound biopsy results at more definitive histology? Urol Oncol. 2014; 32:741–747.
47. Shukla-Dave A, Hricak H, Akin O, Yu C, Zakian KL, Udo K, et al. Preoperative nomograms incorporating magnetic resonance imaging and spectroscopy for prediction of insignificant prostate cancer. BJU Int. 2012; 109:1315–1322.
48. Thompson JE, Moses D, Shnier R, Brenner P, Delprado W, Ponsky L, et al. Multiparametric magnetic resonance imaging guided diagnostic biopsy detects significant prostate cancer and could reduce unnecessary biopsies and over detection: a prospective study. J Urol. 2014; 192:67–74.
49. Kim TH, Jeong JY, Lee SW, Kim CK, Park BK, Sung HH, et al. Diffusion-weighted magnetic resonance imaging for prediction of insignificant prostate cancer in potential candidates for active surveillance. Eur Radiol. 2015; 25:1786–1792.
50. Park BH, Jeon HG, Choo SH, Jeong BC, Seo SI, Jeon SS, et al. Role of multiparametric 3.0-Tesla magnetic resonance imaging in patients with prostate cancer eligible for active surveillance. BJU Int. 2014; 113:864–870.
51. Turkbey B, Mani H, Aras O, Ho J, Hoang A, Rastinehad AR, et al. Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology. 2013; 268:144–152.
52. Morgan VA, Riches SF, Thomas K, Vanas N, Parker C, Giles S, et al. Diffusion-weighted magnetic resonance imaging for monitoring prostate cancer progression in patients managed by active surveillance. Br J Radiol. 2011; 84:31–37.
53. Dianat SS, Carter HB, Pienta KJ, Schaeffer EM, Landis PK, Epstein JI, et al. Magnetic resonance-invisible versus magnetic resonance-visible prostate cancer in active surveillance: a preliminary report on disease outcomes. Urology. 2015; 85:147–153.
54. Mullins JK, Bonekamp D, Landis P, Begum H, Partin AW, Epstein JI, et al. Multiparametric magnetic resonance imaging findings in men with low-risk prostate cancer followed using active surveillance. BJU Int. 2013; 111:1037–1045.
55. Siddiqui MM, Truong H, Rais-Bahrami S, Stamatakis L, Logan J, Walton-Diaz A, et al. Clinical implications of a multiparametric magnetic resonance imaging based nomogram applied to prostate cancer active surveillance. J Urol. 2015; 193:1943–1949.
56. D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998; 280:969–974.
57. Jeong IG, Lim JH, You D, Kim MH, Choi HJ, Kim JK, et al. Incremental value of magnetic resonance imaging for clinically high risk prostate cancer in 922 radical prostatectomies. J Urol. 2013; 190:2054–2060.
58. Somford DM, Hamoen EH, Futterer JJ, van Basten JP, Hulsbergen-van de Kaa CA, Vreuls W, et al. The predictive value of endorectal 3 Tesla multiparametric magnetic resonance imaging for extraprostatic extension in patients with low, intermediate and high risk prostate cancer. J Urol. 2013; 190:1728–1734.
59. Pinaquy JB, De Clermont-Galleran H, Pasticier G, Rigou G, Alberti N, Hindie E, et al. Comparative effectiveness of [(18) F]-fluorocholine PET-CT and pelvic MRI with diffusion-weighted imaging for staging in patients with high-risk prostate cancer. Prostate. 2015; 75:323–331.
60. Park BH, Jeon HG, Jeong BC, Seo SI, Lee HM, Choi HY, et al. Influence of magnetic resonance imaging in the decision to preserve or resect neurovascular bundles at robotic assisted laparoscopic radical prostatectomy. J Urol. 2014; 192:82–88.
61. Feng TS, Sharif-Afshar AR, Smith SC, Miller J, Nguyen C, Li Q, et al. Multiparametric magnetic resonance imaging localizes established extracapsular extension of prostate cancer. Urol Oncol. 2015; 33:109.e15–109.e22.
62. Van den Bergh L, Lerut E, Haustermans K, Deroose CM, Oyen R, Isebaert S, et al. Final analysis of a prospective trial on functional imaging for nodal staging in patients with prostate cancer at high risk for lymph node involvement. Urol Oncol. 2015; 33:109.e23–109.e31.
63. Pasoglou V, Larbi A, Collette L, Annet L, Jamar F, Machiels JP, et al. One-step TNM staging of high-risk prostate cancer using magnetic resonance imaging (MRI): toward an upfront simplified "all-in-one" imaging approach? Prostate. 2014; 74:469–477.
64. Alfarone A, Panebianco V, Schillaci O, Salciccia S, Cattarino S, Mariotti G, et al. Comparative analysis of multiparametric magnetic resonance and PET-CT in the management of local recurrence after radical prostatectomy for prostate cancer. Crit Rev Oncol Hematol. 2012; 84:109–121.
65. Linder BJ, Kawashima A, Woodrum DA, Tollefson MK, Karnes J, Davis BJ, et al. Early localization of recurrent prostate cancer after prostatectomy by endorectal coil magnetic resonance imaging. Can J Urol. 2014; 21:7283–7289.
66. Roy C, Foudi F, Charton J, Jung M, Lang H, Saussine C, et al. Comparative sensitivities of functional MRI sequences in detection of local recurrence of prostate carcinoma after radical prostatectomy or external-beam radiotherapy. AJR Am J Roentgenol. 2013; 200:W361–W368.
67. Wassberg C, Akin O, Vargas HA, Shukla-Dave A, Zhang J, Hricak H. The incremental value of contrast-enhanced MRI in the detection of biopsy-proven local recurrence of prostate cancer after radical prostatectomy: effect of reader experience. AJR Am J Roentgenol. 2012; 199:360–366.
68. Panebianco V, Barchetti F, Sciarra A, Musio D, Forte V, Gentile V, et al. Prostate cancer recurrence after radical prostatectomy: the role of 3-T diffusion imaging in multi-parametric magnetic resonance imaging. Eur Radiol. 2013; 23:1745–1752.
69. Cha D, Kim CK, Park SY, Park JJ, Park BK. Evaluation of suspected soft tissue lesion in the prostate bed after radical prostatectomy using 3T multiparametric magnetic resonance imaging. Magn Reson Imaging. 2015; 33:407–412.
70. Kitajima K, Murphy RC, Nathan MA, Froemming AT, Hagen CE, Takahashi N, et al. Detection of recurrent prostate cancer after radical prostatectomy: comparison of 11C-choline PET/CT with pelvic multiparametric MR imaging with endorectal coil. J Nucl Med. 2014; 55:223–232.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download