Abstract
Tissue transfer techniques are an essential part of the reconstructive urologist's armamentarium. Flaps and graft techniques are widely used in genital and urethral reconstruction. A graft is tissue that is moved from a donor site to a recipient site without its native blood supply. The main types of grafts used in urology are full thickness grafts, split thickness skin grafts and buccal mucosa grafts. Flaps are transferred from the donor site to the recipient site on a pedicle containing its native blood supply. Flaps can be classified based on blood supply, elevation methods or the method of transfer. The most used flaps in urology include penile, preputial, and scrotal skin. We review the various techniques used in reconstructive urology and the outcomes of these techniques.
Expertise in tissue transfer techniques is essential in urologic reconstruction. Tissue can be transferred as either a flap or a graft, and a variety of these techniques have been used in urologic surgery for over fifty years [12]. The choice of tissue transfer technique used is based on the characteristics of the defect, potential donor site, and the patient's global health status. Cosmesis, functionality of the site to be reconstructed, and donor site morbidity must be considered as well.
The two major areas in urology requiring tissue transfer techniques are in addressing genital skin defects and urethral strictures. Other processes that may require tissue transfer include hypospadias, buried penis, lymphedema, genital trauma, and soft tissue infections [345678]. Furthermore, tissue transfer techniques are now being applied to other areas of urologic reconstruction, such as in ureteral reconstruction. In this review, we describe the tissue transfer techniques used most commonly in urologic reconstruction.
There are three main steps necessary for successful reconstruction with a graft: preparation of the recipient site for the graft, harvest of the graft and placement of the graft at the recipient site. Depending on the cause of the defect, the recipient site may need debridement of necrotic tissue or excision of fibrosis or other abnormalities before it is ready to receive the graft [910]. Harvest of the graft can be performed at the same time as the preparation of the recipient site. Due to the possibility of graft contraction, the graft should be significantly larger than the recipient site [9]. The graft can then be placed via any of a variety of techniques (described below).
Unique to graft reconstruction is that tissue is removed from a donor site and transferred to a recipient site without its native blood supply. Blood supply is re-established through two processes: imbibition and inosculation. In the first 48 hours, graft survival is dependent on imbibition where nutrients and waste are passed between the recipient and graft tissue through passive diffusion [11]. From 48 hours to 1 week after surgery, engraftment continues with inosculation, a process in which there is capillary in-growth of host vasculature [11]. Both of these steps are optimized with rapid onset, well-vascularized recipient bed, and good apposition and immobilization of the graft. Reinnervation of the graft is variable and occurs over months to years. Nerves regenerate into the graft from both the margins and the wound bed. Success of reinnervation correlates with the amount of nerve tissue present in the graft [12]. Major causes of graft failure are fluid accumulation in the graft bed, such as hematoma or seroma, infection, and shearing of the graft. Graft meshing is a common technique to allow egress of fluid and improve graft take. The "pie-crusting" technique, in which small perforations are made in the graft, can be used instead of meshing for improved cosmesis. This technique allows for egress of fluid, and leaves little noticeable scarring.
A variety of graft substrate, including skin, bladder, colon and buccal mucosa, has been used in urologic reconstruction [131415]. Regardless of tissue type, grafts may be characterized as either split thickness or full thickness [16].
Full thickness skin grafts (FTSG) include the entire epidermis and dermis. Due to the elastin content of the dermis, FTSGs are more prone to primary contraction, which is the amount of contraction that occurs when the graft is first harvested, than split thickness skin grafts (STSG). However in comparison to STSGs, FTSGs are less prone to secondary contraction, which is the contraction that occurs after the graft is transferred to the recipient site. They are more fastidious and resistant to mechanical trauma. FTSGs can also stretch with the growth of the patient [9]. The dermis contains sebaceous glands, sweat glands and hair follicles. The presence of glandular tissue may prevent drying and cracking of the grafted skin. Of note, since FTSGs include hair follicles, it is important to take the graft from a hairless area if the recipient site is supposed to be hairless. This consideration is especially important in the urethra, as hair in the urethra may result in stones. Additionally, during harvesting of a FTSG, it is important to remove any subcutaneous fat from the graft to increase likelihood of graft take [91718]. There are various areas from which a FTSG can be harvested safely and used in reconstructive urology with good outcomes, including hairless areas of the abdominal wall or inguinal region [10181920], postauricular skin [21] and the prepuce [22].
STSGs are made up of epidermis and only a portion of the dermis. The graft is harvested at varying depths with a dermatome. STSGs have decreased metabolic demand in comparison to full thickness grafts. They tend to have better take than FTSGs in contaminated wounds [23]. Further, since STSGs have little dermis, the risk of recurrent lymphedema is less compared to FTSGs if used in reconstruction after removal of lymphedematous skin [24]. STSGs are hairless, which improves cosmesis in penile reconstruction and functionality in urethral repair. Furthermore, while a FTSGs leaves a full thickness defect at the donor site requiring closure, STSGs harvest leaves a site that heals with wound care [9]. Common donor sites for STSGs in reconstructive urology are the lateral or medial thigh [25262728].
Full thickness buccal mucosa grafts (BMG) are commonly used in urethral reconstruction. BMGs have secretory epithelium, which closely resembles urethral epithelium, rather than the cornified epithelium in skin grafts. BMGs have the advantageous contractile properties of FTSGs, but are hairless. A BMG has a robust microvascular network that yields better circulation than a FTSG [29]. Buccal mucosa is readily available, strong and resists infection [30]. Furthermore, the harvest site is concealed.
A BMG is harvested from the inner cheek or the inner lip. Care must be taken to avoid Stensen's duct and not to get too close to the lip, to prevent mouth contractures. Morbidity in BMG harvest is minimal, with a complication rate reported at 3%-4% [31]. The most common complications include donor site scarring, perioral sensory defect and jaw opening impairment [2932]. Oral mucosa grafts can also be harvested from the posterior aspect of the tongue (lingual). Lingual mucosa grafts have similar tissue characteristics as BMGs and have also been used in urethral reconstruction with comparable results [3334].
Rather than rely on the recipient site for survival, flaps are transferred on a pedicle containing native blood supply. For this reason, flaps can be used in a wound that cannot otherwise support a graft.
Flaps can be classified based on their blood supply, elevation method, or their method of transfer. The blood supply may be random or axial. Random flaps do not have a defined blood supply and rely on undefined vasculature in the pedicle to be functional. Typically, random flaps depend on the dermal and subdermal plexus [35]. Axial flaps depend on a specific blood vessel with a known distribution [16]. Axial flaps are further subdivided by the structure carrying the blood supply. For instance, in musculocutaneous flaps, the underlying muscle carries the blood supply, while in fasciocutaneous flaps the blood supply is carried by the fascia. Musculocutaneous flaps contain dermal and subcutaneous tissue along with the musculocutaneous perforator vessels and the perforating cutaneous branches of the muscular vessels [35]. Fasciocutaneous flaps are composed of skin, subcutaneous tissue, and underlying fascia [35].
Flaps are also characterized by their method of elevation and various methods of flap transfer [13]. A peninsula flap is one in which the vascular and cutaneous connections are left intact. They may be transferred as advancement flaps, in which the graft is moved in a parallel direction to its pedicle, or rotational flaps, in which the graft is transferred perpendicular to the long axis of its pedicle. An island flap is one in which the skin is divided but the vascular connections are maintained. Island flaps provide excellent blood supply, especially in comparison to advancement flaps, which are at risk for decreased blood flow to the most distant areas from the pedicle [36]. A free flap is one in which the tissue and vessels are excised from the donor site, and the vessels are anastomosed to suitable vessels at the recipient site to re-establish blood flow [16]. Both musculocutaneous and fasciocutaneous flaps can be transferred via the free or island flap technique [35].
In parallel with graft harvest, flaps used for reconstruction of the penis or urethra should be hairless. Flaps used for penile reconstruction should be thicker than those used for urethral reconstruction [13]. Flaps can be taken from redundancy of penile skin (both ventral and dorsal), hairless scrotal skin, the gracilis muscle (as a musculocutaneous flap), and as free flaps (usually from the forearm or upper arm) [1316]. Scrotal fasciocutaneous flaps can be based on the posterior branches of the superficial external pudendal artery or the perineal artery.
Genital skin loss occurs secondary to various processes such as Fournier's gangrene, trauma, and burns [347]. Furthermore reconstruction may be desired for genital lymphedema or after surgical resection of malignancies such as penile carcinoma [437].
Skin of the penis is thin, hairless and flexible. Any tissue used to replace penile skin must be able to expand with erections and be durable enough to withstand sexual activity. Scrotal skin flaps may be used for penile coverage, but due to concern about poor cosmesis, skin grafts are the preferred method of reconstruction.
FTSGs or STSGs may be used depending on a number of considerations. FTSGs have more donor morbidity, but higher elasticity and less contracture in comparison to STSGs [2037]. FTSGs may also contain hair, which may be a cosmetic problem.
STSGs are easy to harvest and have very high rates of graft take with excellent cosmetic results [3839] (Fig. 1). The donor sites are usually from the lateral thigh. STSGs on the penis allow normal mobility of the skin overlying the corpora. Although STSGs have significant primary contraction, patients are usually able to have erections sufficient for intercourse [26]. Other studies focusing on STSGs for genital skin loss showed improvement of the clinical condition, good cosmesis and improved quality of life [2640]. STSGs may also be preferable in reconstruction after resection for lymphedema, due to the fact that both flaps and FTSGs carry lymphatics and risk recurrent lymphedema [13]. Of note, meshed grafts on the penis may result in poor cosmesis.
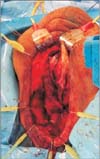
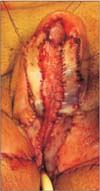
The glans may require reconstruction in cases of trauma or surgical intervention for lichen sclerosis, penile carcinoma in situ and penile carcinoma. Glans resurfacing, in which the affected skin is excised, may be performed and a STSG is used to create a new glans [41] (Figs. 2, 3, 4). Urethral mucosa flap may also be used to provide coverage [42].
Partial scrotal skin defects can often be closed primarily due to the redundancy of scrotal skin. Scrotal defects that cannot be closed primarily may be reconstructed with rotational skin flaps or STSGs. Rotational thigh flaps have been used for reconstruction of large scrotal defects with the advantage of a sensate and hair baring reconstruction [4344]. Flaps also avoid the issues associated with graft take. Meshed STSGs are often the grafts of choice for large scrotal defects. Meshed STSGs closely resembles the natural scrotal rugae upon healing and the meshing also allows for the drainage of fluid from the graft. The tunica vaginalis of the testes are reliable graft beds. However, the grafts are insensate and are unable to provide thermoregulation to optimize spermatogenesis [37].
While primary anastomosis is an effective means of urethral reconstruction for shorter defects of the urethra with 93% success rate [45], abnormalities longer than 2 cm may require substitution urethroplasty [30]. In particular, in the penile urethra, excision and primary anastomosis often results in penile curvature [46], making augmentation urethroplasty with a flap or graft desirable. Multiple techniques using various flaps and grafts have been used. FTSGs and penile skin fasciocutaneous flaps were the mainstay of treatment until the emergence of BMG urethroplasty in 1990s. For the bulbar urethra, the decision for primary anastomosis versus urethroplasty with tissue transfer is primarily based on stricture length [46].
The current gold standard for augmentation urethroplasty is the BMG [46]. However, STSGs and FTSGs may still be required for long urethral abnormalities or in patients with contraindications to buccal mucosa harvest, such as those with leukoplakia, systemic skin disease of the oral cavity or long history of tobacco use [47]. Extragenital skin grafts may also be considered in patients who have genital skin diseases such as lichen sclerosis, which prevents the use of genital skin for flap repair.
Both STSGs and FTSGs have been used in urethral reconstruction. STSGs, taken from the right thigh, have excellent take rates and cosmetic results are satisfactory [2848]. Dalpiaz et al. [28] reported a 93% success rate at mean follow up of 32.43 months using extragenital STSGs for single stage urethroplasty in patients with contraindications to BMG harvest. FTSGs taken from abdominal, penile, and posterior auricular skin, have also been used in urethroplasty [192149]. Hussein et al. [49] reported a 72.3% success rate for penile graft urethroplasty. Abdominal wall grafts have also been used in cases where genital skin grafts and buccal grafts were contraindicated. Liu et al. [19] reported a stricture recurrence rate of 53.8% in patients who underwent urethroplasty with abdominal wall skin graft versus 24% in those who underwent other types of graft urethroplasty. However, these patients also had significantly longer strictures, increased history of failed urethroplasty, and increased proportion of patients with lichen sclerosis.
Buccal mucosa grafting for urethral stricture reconstruction was first pioneered in the mid 1990s [50], and has revolutionized the management of urethral strictures [51]. BMGs, with harvest from both cheeks and the lower lip, have been successfully used to repair urethral abnormalities up to 17 cm long [5253].
Technique for BMG harvest begins with preparation of the inner cheek. Stay sutures may be placed to retract the cheek and lip and keep the graft on stretch. Stensen's duct is identified and avoided. A lidocaine and epinephrine solution is injected submucosally for both hemostasis and hydrodissection. The edges of the mucosa are then incised, and the graft is dissected away from the underlying buccinators muscle. Following dissection, the graft is defatted and then stored in saline until it is needed. The intraoral defect can be packed until hemostasis is achieved or closed with a running suture [5455].
There has been extensive debate regarding the optimal position of grafts used in urethroplasty. Various techniques have been described regarding the placement of the graft as a ventral or dorsal onlay. Morey and McAninch [51] first reported the ventral onlay technique of BMG urethroplasty in 1996. The strictured segment of urethra is incised ventrally until urethra of adequate caliber is reached proximally and distally. An appropriately sized graft is sutured into the defect in a watertight fashion. A spongioplasty is then performed over the graft. Ventral onlay grafts may be easier to perform in the proximal part of the bulbar urethra, where the spongiosum is thicker and better vascularized [56].
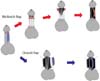
The dorsal onlay technique was initially described by Barbagli et al. [57] in 1996. In this technique, the urethra is circumferentially mobilized from the cavernous bodies and the urethrostomy is made on the dorsal wall of the urethra. The graft is then fixed onto the corpora cavernosa underlying the urethrostomy; the edges of the urethrostomy are then sutured to the edges of the now spread-fixed graft. This approach allows the surgeon to fix the graft to the corporeal bodies, which may promote graft survival and prevent retraction of the graft. Furthermore, the dorsal approach obviates concern for graft sacculation as may occur in a ventral graft [58]. Kulkarni et al. [59] presented a modification of the dorsal onlay technique with one sided mobilization rather than circumferential mobilization; this technique preserves the lateral vascular supply to the urethra (Fig. 5). Other approaches used are lateral onlay and double overlapping graft techniques.
An inlay technique was described by Asopa et al. [60]. In this technique a ventral urethrostomy and a dorsal incision are made in the urethral plate. The graft is then sutured into the incised plate, thus augmenting the strictured segment of urethra [60]. The ventral urethrostomy is then closed.
An onlay technique can be used in combination with an excision in a technique known as an augmented anastomotic urethroplasty. In this procedure, the obliterative segment is excised and one side of the urethra is reanastomosed. The urethra is then spatulated opposite the anastomosis and a graft is then placed to augment the size of the anastomosis. Guralnick and Webster [61], showed that augmented anastomosis has greater than 90% success.
The majority of urethral reconstructions can be performed in one stage. A two-stage repair may be advantageous in cases requiring extensive reconstruction, such as that for hypospadias cases and urethral strictures due to lichen sclerosis [536263]. In a two-stage procedure, the damaged area is opened, and a new urethral plate is created with grafts (Fig. 6). Once this has healed, usually after 6 months, the new urethral plate is tubularized into a neourethra.
Flap reconstruction can be used in combination with other tissue transfer techniques, such as onlay grafts, as needed.
Penile and preputial skin island flaps are the most commonly used in flap reconstruction of the anterior urethra. These are fasciocutaneous flaps that rely on the tunica dartos to provide vascular supply. Preputial skin is well suited for urethral reconstruction because it is thin and hairless. Skin on the distal penile shaft is typically hairless and is well suited for urethral reconstruction whereas skin on the mid to proximal penile shaft may be hair bearing to varying degrees.
The penile skin flap may be harvested in transverse or longitudinal fashion and then rotated into the urethral defect. Orandi described a longitudinal penile skin island flap with a lateral pedicle [2] (Fig. 7, bottom). The skin island is harvested from the ventral aspect of the penis and is dissected from medial to lateral, leaving the flap with a lateral pedicle. McAninch, on the other hand, described a transverse preputial skin flap harvested in circumferential fashion [6465] (Fig. 7, top). During reconstruction, a circular flap is made using the prepuce in uncircumcised individuals or distal shaft skin in circumcised individuals. These flaps can provide 13-15 cm of tissue, making them ideal for long strictures [65]. Onlay flaps are preferable to tubularized flaps due to lower failure rates [66].
Dubey et al. [67], in a randomized prospective study comparing dorsal onlay BMG urethroplasty and penile skin flap urethroplasty, reported that success rates at two-year follow-up were slightly in favor of BMG (89.9% vs. 85.6%), but the difference was not statistically significant. However, on longer follow-up, graft urethroplasty was more successful. On retrospective analysis, overall success rate of graft urethroplasty was significantly better than flap urethroplasty with 80% compared to 67%, respectively [68]. Additionally, long-term data has found that at fifteen-year follow-up flap urethroplasty has a 42% stricture recurrence rate and 33% complication rate [45].
The use of BMGs in the open repair of ureteral strictures has been reported in several case series starting in 1999 [6970717273]. The graft has been successfully placed both as an onlay and as a tubularized segment in cases where end-to-end anastomosis was not feasible. This may be a useful alternative technique for ureteral reconstruction, particularly in cases of long proximal ureteral strictures, which may otherwise require ileal replacement or renal autotransplant. Abnormal tissue is resected until healthy margins are obtained. In a similar fashion to the urethroplasty technique, the BMG is placed as a dorsal or ventral onlay. A ureteral stent is placed and the ureter is wrapped most often with omentum to augment vascularity. There have been few reported complications and recurrence rate on follow up ranging up to 85 months is low [73]. While these reported cases have been performed with the open technique, we have performed BMG ureteroplasty using a robotic assisted approach with success on short term follow-up [7475] (Figs. 8, 9).
Figures and Tables
Fig. 1
Patient with Fournier's gangrene. Split thickness skin graft was used for scrotal reconstruction.
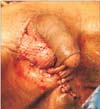
Fig. 3
Split thickness skin graft on reconstructed glans after partial penectomy, one week after surgery.
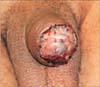
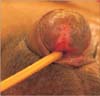
Fig. 4
Glans reconstruction using split thickness skin graft after partial penectomy, two months after reconstruction.
References
1. Presman D, Greenfield DL. Reconstruction of the perineal urethra with a free full-thickness skin graft from the prepuce. J Urol. 1953; 69:677–680.
2. Orandi A. One-stage urethroplasty. Br J Urol. 1968; 40:717–719.
3. Castro RB, Oliveira AB, Favorito LA. Utilization of skin flap for reconstruction of the genitalia after an electric burn. Int Braz J Urol. 2006; 32:68–69.
4. Ferreira PC, Reis JC, Amarante JM, Silva AC, Pinho CJ, Oliveira IC, et al. Fournier's gangrene: a review of 43 reconstructive cases. Plast Reconstr Surg. 2007; 119:175–184.
5. Garaffa G, Christopher N, Ralph DJ. The management of genital lymphoedema. BJU Int. 2008; 102:480–484.
6. Tang SH, Kamat D, Santucci RA. Modern management of adult-acquired buried penis. Urology. 2008; 72:124–127.
7. Mokhless IA, Abdeldaeim HM, Rahman A, Zahran M, Safwat A. Penile advancement and lengthening for the management of post-circumcision traumatic short penis in adolescents. Urology. 2010; 76:1483–1487.
8. Higuchi TT, Yamaguchi Y, Wood HM, Angermeier KW. Evaluation and treatment of adult concealed penis. Curr Urol Rep. 2012; 13:277–284.
9. Thakar HJ, Dugi DD 3rd. Skin grafting of the penis. Urol Clin North Am. 2013; 40:439–448.
10. Meeks JJ, Erickson BA, Gonzalez CM. Full-thickness abdominal skin graft for long-segment urethral stricture reconstruction. Int Braz J Urol. 2008; 34:602–607.
11. Greenwood J, Amjadi M, Dearman B, Mackie I. Real-time demonstration of split skin graft inosculation and integra dermal matrix neovascularization using confocal laser scanning microscopy. Eplasty. 2009; 9:e33.
12. Waris T, Rechardt L, Kyösola K. Reinnervation of human skin grafts: a histochemical study. Plast Reconstr Surg. 1983; 72:439–447.
13. Jordan GH. Penile reconstruction, phallic construction, and urethral reconstruction. Urol Clin North Am. 1999; 26:1–13.
14. Kinkead TM, Borzi PA, Duffy PG, Ransley PG. Long-term followup of bladder mucosa graft for male urethral reconstruction. J Urol. 1994; 151:1056–1058.
15. Xu YM, Qiao Y, Sa YL, Zhang J, Fu Q, Song LJ. Urethral reconstruction using colonic mucosa graft for complex strictures. J Urol. 2009; 182:1040–1043.
16. Jordan GH. Principles of tissue transfer techniques in urethral reconstruction. Urol Clin North Am. 2002; 29:267–275.
17. Taifour Suliman M. A simple method to facilitate full-thickness skin graft harvest. Burns. 2009; 35:87–88.
18. Meeks JJ, Erickson BA, Fetchev P, Crawford SE, Fine NA, Gonzalez CM. Urethroplasty with abdominal skin grafts for long segment urethral strictures. J Urol. 2010; 183:1880–1884.
19. Liu JS, Han J, Said M, Hofer MD, Fuchs A, Ballek N, et al. Long-term outcomes of urethroplasty with abdominal wall skin grafts. Urology. 2015; 85:258–262.
20. Thompson JH, Zmaj P, Cummings JM, Steinhardt GF. An approach for using full thickness skin grafts for complex penile surgeries in children. J Urol. 2006; 175:1869–1871.
21. Manoj B, Sanjeev N, Pandurang PN, Jaideep M, Ravi M. Postauricular skin as an alternative to oral mucosa for anterior onlay graft urethroplasty: a preliminary experience in patients with oral mucosa changes. Urology. 2009; 74:345–348.
22. Yildirim S, Akan M, Akoz T, Tanoglu B. The preputium: an overlooked skin graft donor site. Ann Plast Surg. 2001; 46:630–634.
23. Vincent MP, Horton CE, Devine CJ Jr. An evaluation of skin grafts for reconstruction of the penis and scrotum. Clin Plast Surg. 1988; 15:411–424.
24. Malloy TR, Wein AJ, Gross P. Scrotal and penile lymphedema: surgical considerations and management. J Urol. 1983; 130:263–265.
25. Black PC, Friedrich JB, Engrav LH, Wessells H. Meshed unexpanded split-thickness skin grafting for reconstruction of penile skin loss. J Urol. 2004; 172:976–979.
26. Morey AF, Meng MV, McAninch JW. Skin graft reconstruction of chronic genital lymphedema. Urology. 1997; 50:423–426.
27. Carr LK, MacDiarmid SA, Webster GD. Treatment of complex anterior urethral stricture disease with mesh graft urethroplasty. J Urol. 1997; 157:104–108.
28. Dalpiaz O, Kerschbaumer A, Pelzer A, Radmayr C, Gozzi C, Horninger W, et al. Single-stage dorsal inlay split-skin graft for salvage anterior urethral reconstruction. BJU Int. 2008; 101:1565–1570.
29. Liu Y, Zhuang L, Ye W, Ping P, Wu M. One-stage dorsal inlay oral mucosa graft urethroplasty for anterior urethral stricture. BMC Urol. 2014; 14:35.
30. Levine LA, Strom KH, Lux MM. Buccal mucosa graft urethroplasty for anterior urethral stricture repair: evaluation of the impact of stricture location and lichen sclerosus on surgical outcome. J Urol. 2007; 178:2011–2015.
31. Castagnetti M, Ghirardo V, Capizzi A, Andretta M, Rigamonti W. Donor site outcome after oral mucosa harvest for urethroplasty in children and adults. J Urol. 2008; 180:2624–2628.
32. Wood DN, Allen SE, Andrich DE, Greenwell TJ, Mundy AR. The morbidity of buccal mucosal graft harvest for urethroplasty and the effect of nonclosure of the graft harvest site on postoperative pain. J Urol. 2004; 172:580–583.
33. Simonato A, Gregori A, Lissiani A, Galli S, Ottaviani F, Rossi R, et al. The tongue as an alternative donor site for graft urethroplasty: a pilot study. J Urol. 2006; 175:589–592.
34. Barbagli G, De Angelis M, Romano G, Ciabatti PG, Lazzeri M. The use of lingual mucosal graft in adult anterior urethroplasty: surgical steps and short-term outcome. Eur Urol. 2008; 54:671–676.
35. Nakajima H, Fujino T, Adachi S. A new concept of vascular supply to the skin and classification of skin flaps according to their vascularization. Ann Plast Surg. 1986; 16:1–19.
36. Kimyai-Asadi A, Goldberg LH. Island pedicle flap. Dermatol Clin. 2005; 23:113–127.
37. Garaffa G, Sansalone S, Ralph DJ. Penile reconstruction. Asian J Androl. 2013; 15:16–19.
38. Stokes TH, Follmar KE, Silverstein AD, Weizer AZ, Donatucci CF, Anderson EE, et al. Use of negative-pressure dressings and split-thickness skin grafts following penile shaft reduction and reduction scrotoplasty in the management of penoscrotal elephantiasis. Ann Plast Surg. 2006; 56:649–653.
39. Gillett MD, Rathbun SR, Husmann DA, Clay RP, Kramer SA. Split-thickness skin graft for the management of concealed penis. J Urol. 2005; 173:579–582.
40. Modolin M, Mitre AI, da Silva JC, Cintra W, Quagliano AP, Arap S, et al. Surgical treatment of lymphedema of the penis and scrotum. Clinics (Sao Paulo). 2006; 61:289–294.
41. Hadway P, Corbishley CM, Watkin NA. Total glans resurfacing for premalignant lesions of the penis: initial outcome data. BJU Int. 2006; 98:532–536.
42. Belinky JJ, Cheliz GM, Graziano CA, Rey HM. Glanuloplasty with urethral flap after partial penectomy. J Urol. 2011; 185:204–206.
43. McDougal WS. Scrotal reconstruction using thigh pedicle flaps. J Urol. 1983; 129:757–759.
44. Lin CT, Chang SC, Chen SG, Tzeng YS. Reconstruction of perineoscrotal defects in Fournier's gangrene with pedicle anterolateral thigh perforator flap. ANZ J Surg. 2014; 2014:Jul. 24. [Epub]. DOI: 10.1111/ans.12782.
45. Andrich DE, Dunglison N, Greenwell TJ, Mundy AR. The long-term results of urethroplasty. J Urol. 2003; 170:90–92.
46. Lee YJ, Kim SW. Current management of urethral stricture. Korean J Urol. 2013; 54:561–569.
47. Splieth CH, Sumnig W, Bessel F, John U, Kocher T. Prevalence of oral mucosal lesions in a representative population. Quintessence Int. 2007; 38:23–29.
48. Ehrlich RM, Alter G. Split-thickness skin graft urethroplasty and tunica vaginalis flaps for failed hypospadias repairs. J Urol. 1996; 155:131–134.
49. Hussein MM, Moursy E, Gamal W, Zaki M, Rashed A, Abozaid A. The use of penile skin graft versus penile skin flap in the repair of long bulbo-penile urethral stricture: a prospective randomized study. Urology. 2011; 77:1232–1237.
50. el-Kasaby AW, Fath-Alla M, Noweir AM, el-Halaby MR, Zakaria W, el-Beialy MH. The use of buccal mucosa patch graft in the management of anterior urethral strictures. J Urol. 1993; 149:276–278.
51. Morey AF, McAninch JW. When and how to use buccal mucosal grafts in adult bulbar urethroplasty. Urology. 1996; 48:194–198.
52. Dubey D, Kumar A, Mandhani A, Srivastava A, Kapoor R, Bhandari M. Buccal mucosal urethroplasty: a versatile technique for all urethral segments. BJU Int. 2005; 95:625–629.
53. Meeks JJ, Erickson BA, Gonzalez CM. Staged reconstruction of long segment urethral strictures in men with previous pediatric hypospadias repair. J Urol. 2009; 181:685–689.
54. Cohen SD, Armenakas NA, Light DM, Fracchia JA, Glasberg SB. Single-surgeon experience of 87 buccal mucosal graft harvests. Plast Reconstr Surg. 2012; 130:101–104.
55. Morey AF, McAninch JW. Technique of harvesting buccal mucosa for urethral reconstruction. J Urol. 1996; 155:1696–1697.
56. Barbagli G, Palminteri E, Guazzoni G, Montorsi F, Turini D, Lazzeri M. Bulbar urethroplasty using buccal mucosa grafts placed on the ventral, dorsal or lateral surface of the urethra: are results affected by the surgical technique? J Urol. 2005; 174:955–957.
57. Barbagli G, Selli C, Tosto A, Palminteri E. Dorsal free graft urethroplasty. J Urol. 1996; 155:123–126.
58. Iselin CE, Webster GD. Dorsal onlay graft urethroplasty for repair of bulbar urethral stricture. J Urol. 1999; 161:815–818.
59. Kulkarni S, Barbagli G, Sansalone S, Lazzeri M. One-sided anterior urethroplasty: a new dorsal onlay graft technique. BJU Int. 2009; 104:1150–1155.
60. Asopa HS, Garg M, Singhal GG, Singh L, Asopa J, Nischal A. Dorsal free graft urethroplasty for urethral stricture by ventral sagittal urethrotomy approach. Urology. 2001; 58:657–659.
61. Guralnick ML, Webster GD. The augmented anastomotic urethroplasty: indications and outcome in 29 patients. J Urol. 2001; 165:1496–1501.
62. Dubey D, Sehgal A, Srivastava A, Mandhani A, Kapoor R, Kumar A. Buccal mucosal urethroplasty for balanitis xerotica obliterans related urethral strictures: the outcome of 1 and 2-stage techniques. J Urol. 2005; 173:463–466.
63. Zheng DC, Yao HJ, Cai ZK, Da J, Chen Q, Chen YB, et al. Two-stage urethroplasty is a better choice for proximal hypospadias with severe chordee after urethral plate transection: a single-center experience. Asian J Androl. 2015; 17:94–97.
64. McAninch JW. Reconstruction of extensive urethral strictures: circular fasciocutaneous penile flap. J Urol. 1993; 149:488–491.
65. Carney KJ, McAninch JW. Penile circular fasciocutaneous flaps to reconstruct complex anterior urethral strictures. Urol Clin North Am. 2002; 29:397–409.
66. Srivastava A, Vashishtha S, Singh UP, Srivastava A, Ansari MS, Kapoor R, et al. Preputial/penile skin flap, as a dorsal onlay or tubularized flap: a versatile substitute for complex anterior urethral stricture. BJU Int. 2012; 110(11 Pt C):E1101–E1108.
67. Dubey D, Vijjan V, Kapoor R, Srivastava A, Mandhani A, Kumar A, et al. Dorsal onlay buccal mucosa versus penile skin flap urethroplasty for anterior urethral strictures: results from a randomized prospective trial. J Urol. 2007; 178:2466–2469.
68. Barbagli G, Morgia G, Lazzeri M. Retrospective outcome analysis of one-stage penile urethroplasty using a flap or graft in a homogeneous series of patients. BJU Int. 2008; 102:853–860.
69. Agrawal V, Dassi V, Andankar MG. Buccal mucosal graft onlay repair for a ureteric ischemic injury following a pyeloplasty. Indian J Urol. 2010; 26:120–122.
70. Badawy AA, Abolyosr A, Saleem MD, Abuzeid AM. Buccal mucosa graft for ureteral stricture substitution: initial experience. Urology. 2010; 76:971–975.
71. Naude JH. Buccal mucosal grafts in the treatment of ureteric lesions. BJU Int. 1999; 83:751–754.
72. Sadhu S, Pandit K, Roy MK, Bajoria SK. Buccal mucosa ureteroplasty for the treatment of complex ureteric injury. Indian J Surg. 2011; 73:71–72.
73. Kroepfl D, Loewen H, Klevecka V, Musch M. Treatment of long ureteric strictures with buccal mucosal grafts. BJU Int. 2010; 105:1452–1455.
74. Marien T, Bjurlin M, Wynia B, Bilbily M, Rao G, Zhao LC, et al. Outcomes of Robotic-Assisted Laparoscopic Upper Urinary Tract Reconstruction: 250 Consecutive Patients. BJU Int. 2015; Feb. 13. [Epub]. DOI: 10.1111/bju.13086.
75. Zhao LC, Yamaguchi Y, Bryk DJ, Maddox MM, Powers MK, Harbin A, et al. MP29-01 multi-institutional study of robotic buccal mucosa graft ureteroplasty: inital results. J Urol. 2015; 193(4S):e339.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download