Abstract
Purpose
Transrectal ultrasound (TRUS)-guided prostate biopsy is the most useful technique for the diagnosis of prostate cancer; however, many patients describe the procedure as uncomfortable and painful. We investigated the effect of the patient's position on pain scales during TRUS-guided prostate biopsy.
Materials and Methods
Between July 2012 and June 2013, a total of 128 consecutive patients who underwent TRUS-guided prostate biopsy were included in this study. Seventy patients underwent the procedure in the lithotomy position performed by a urologist and the other patients (n=58) underwent the procedure in the left lateral decubitus (LLD) position performed by a radiologist. Pain was assessed by using visual analogue scale (VAS) scores from 0 to 10. Using a linear regression model, we analyzed the correlation between pain scale score and clinical variables with a focus on patient position.
Results
No significant differences related to age, body mass index, prostate volume, prostate-specific antigen (PSA), hematuria, pyuria, International Prostate Symptom Score, or the cancer detection rate were observed between the lithotomy and the LLD groups. In the correlation analysis, VAS score showed a significant correlation with diabetes mellitus, PSA level, and lithotomy position (p<0.05). In the multiple linear regression model, VAS score showed a significant correlation with lithotomy position (β=-0.772, p=0.003) and diabetes mellitus (β=-0.803, p=0.033).
In Korea, the incidence rate of prostate cancer was 32.5 per 100,000 during 2012 [1] and has increased annually with the widespread use of prostate-specific antigen (PSA) screening and transrectal ultrasound (TRUS)-guided prostate biopsy. The current trend in TRUS-guided prostate biopsy involves the use of an extended number of biopsy cores (10- to 12-core biopsy), because this scheme results in a higher cancer detection rate [2]. However, as the number of biopsy cores has increased, many patients have described this procedure as uncomfortable and painful [3,4,5].
Several recent studies have reported on relief of pain during prostate biopsy. A randomized trial demonstrated that intrarectal 2% lidocaine gel before prostate biopsy was simple, safe, and effective for providing satisfactory anesthesia during prostate biopsy [6]. In addition, the combination of lidocaine suppository and periprostatic nerve block (PPNB) was effective in controlling pain during TRUS-guided prostate biopsy [7]. Intravenous sedation with an opioid drug was also shown to reduce the discomfort and pain caused by prostate biopsy [8].
Despite the use of these techniques to reduce pain, many patients still experience a considerable degree of pain during TRUS-guided prostate biopsy [9,10]. Some studies have reported that a patient's pain perception during prostate biopsy is related to the patient's position, such as the left lateral decubitus (LLD) or lithotomy position [11,12,13], although this is controversial. Homogeneous data with regard to pain perception during TRUS-guided prostate biopsy are currently sparse, especially in Asian populations.
In this background, we retrospectively investigated the effect of the patient's position (LLD vs. lithotomy position) on pain perception during TRUS-guided prostate biopsy performed as an extended 12-core biopsy with the intrarectal administration of 2% lidocaine gel. We also analyzed the clinical factors that affected pain during TRUS-guided prostate biopsy in a Korean population.
This study was approved by the Institutional Review Board of the Yeungnam University Medical Center (approval No. 2015-03-028). A total of 128 men who underwent TRUS-guided prostate biopsy from July 2012 to June 2013 at a single center were included. The indications for biopsy were an abnormal digital rectal examination or an elevated PSA level. Exclusion criteria were anal or rectal pathologies, chronic pelvic pain syndrome, the presence of urinary tract infection, a history of prostate biopsy, and contraindication for the lithotomy position.
After informed consent was obtained, patients underwent prostate biopsy in the lithotomy position (n=70, Wednesday and Friday) performed by a qualified urologist in an operation room or prostate biopsy in the LLD position (n=58, Tuesday and Thursday) performed by a qualified radiologist in the radiology department. The biopsy date was determined by the patient's preference. All patients underwent 12-core biopsy with the same protocol (2 from the base, 2 from the mid lobe, 1 from the apex, and 1 from the transitional zone). With the use of an 18-gauge 20-cm disposable needle, all biopsies were performed with the Hitachi HIGH VISION 5500 ultrasound machine with the UST-675P prostate probe (Hitachi Aloka Medical Ltd., Tokyo, Japan).
Antibiotic prophylaxis with 250-mg intravenous ciprofloxacin was started on the night before and morning of the procedure. Anticoagulants or antiplatelets were routinely stopped 5 to 7 days before biopsy. All biopsies were performed by using intrarectal application of 10 mL of 2% lidocaine gel 10 minutes before the procedure.
Immediately after the procedure, all patients were asked to complete a questionnaire addressing pain perception that evaluated the level of pain associated with the procedure. The questionnaire was administered by a nonphysician coordinator who was blinded to the procedure. Pain was evaluated by using a 10-point visual analogue scale (VAS) from 0 to 10 (0=painless, 10=intolerable pain).
The clinical variables included age, International Prostate Symptom Score (IPSS), PSA levels, prostate volume, body mass index (BMI), diabetes mellitus (DM), cancer detection rate, microscopic hematuria, and pyuria. Results were expressed as mean and standard deviation for age, IPSS, PSA levels, prostate volume, BMI, cancer detection rate, and VAS. Categorical variables were described by frequency and percentage. Statistical analysis was performed by using the SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Chi-square test and paired t-test were used for comparison of clinical variables between the lithotomy and LLD position. Pearson correlation coefficients and a multiple linear regression model were used for analysis of relationships between clinical variables and VAS. Values of p<0.05 were considered statistically significant.
The patients' mean age and mean PSA levels were 66.9±9.4 years and 15.57±16.89 ng/mL, respectively. The clinical characteristics of the patients are summarized in Table 1. Similar oncologic outcomes of the biopsies were observed between the lithotomy and the LLD positions. The cancer detection rate was 32.8% and 34.4% in the lithotomy and LLD positions, respectively (p=0.941). No statistically significant differences related to other clinical variables, including age, BMI, prostate volume, PSA level, microscopic hematuria, pyuria, IPSS, and DM, were observed between the lithotomy and LLD positions.
Pearson correlation coefficients between VAS score and clinical variables are shown in Table 2. VAS score showed a significant correlation with DM (r=-0.141, p=0.038), PSA level (r=0.183, p=0.019), and lithotomy position (r=-0.225, p=0.011) (Fig. 1). However, there were no significant correlations with age, microscopic hematuria, pyuria, IPSS, prostate cancer, or BMI.
Result of the multiple linear regression analysis for the correlation between VAS score and clinical variables are shown in Table 3. VAS score showed a significant correlation with lithotomy position (β=-0.772, p=0.003) and DM (β=-0.803, p=0.033). The final regression equation was as follows: VAS=3.351-0.772×"lithotomy position"-0.803×"DM" (R2=0.085).
The outcomes of complications af ter biopsy are summarized in Table 4. Rectal bleeding, hematuria, and hematospermia were observed in the lithotomy and LLD positions, all of which resolved within 2 weeks after biopsy without proper treatment. No differences related to complications after biopsy were observed between the positions. Urinary tract infection was not noted in either group.
As the overall number of TRUS-guided prostate biopsies performed for the diagnosis of prostate cancer increases, it is essential for urologists to understand the patient tolerance associated with this procedure. Approximately 65% to 90% of patients report pain of moderate to severe intensity, and 20% do not want to repeat the examination [4]. Pain during TRUS-guided prostate biopsy is generated by transducer insertion and needle penetration in the rectal wall, prostate capsule, and periprostatic tissue [4]. By focusing on this mechanism, several techniques and agents for the alleviation of pain associated with prostate biopsy have been reported.
Among the techniques to relieve pain during TRUS-guided prostate biopsy, PPNB is the most effective method [14]. In a meta-analysis, compared with no anesthesia, placebo, and intrarectal lidocaine gel injection, PPNB significantly reduced the pain arising from TRUS-guided prostate biopsy [15]. However, PPNB can have several complications resulting from repeated injections during the biopsy, systemic lidocaine toxicity, urinary incontinence, and degradation of the image resolution as the result of anesthetic injection [16,17]. In addition, several studies have reported that patients who underwent periprostatic local anesthetic infiltration felt that the pain of probe introduction was as bad as or worse than the needle biopsies themselves [9,10]. Therefore, other methods to relieve pain during TRUS-guided prostate biopsy should be considered.
In the current study, patients in the lithotomy position experienced less pain during TRUS-guided prostate biopsy than did those in the LLD position. Regarding the anatomy of the pelvic floor, we hypothesized that the lithotomy position may result in release of the external anal sphincter as the pelvic floor muscles are relaxed. Some studies have evaluated the activity of the pelvic floor muscle resting tone in different positions. Resende et al. [18] conducted a study comparing three different positions (lithotomy, LLD, and supine position) to evaluate the myoelectrical activity of the pelvic floor muscle resting tone. The LLD position presented a significantly greater myoelectrical signal of pelvic floor resting tone compared with the lithotomy and supine positions. Therefore, we anticipate that pain experienced while undergoing TRUS-guided prostate biopsy from the insertion of the ultrasound probe may be relieved in the lithotomy position.
In addition, we hypothesized that the lithotomy position may be more acceptable than the LLD position regarding communication between patients and physicians. Some studies have reported that pain perception during prostate biopsy was related to the level of anxiety [19,20]. Bruyere et al. [11] published a study comparing the pain experienced during prostate biopsy in two different positions: lithotomy and LLD. The results of that study suggested that the LLD position may be less acceptable and more humiliating than the lithotomy position because of less eye contact and less gentle handling than in the lithotomy position. Although the relationship between the level of anxiety and the patient's position during TRUS-guided prostate biopsy was not analyzed from a psychobehavioral aspect besides the VAS score in our study, we anticipate that the lithotomy position may be more acceptable than the LLD position because patients in the lithotomy position can see the process of the procedure and the physician's movements.
In contrast to our results, Kilciler et al. [12] and Lodeta and Lodeta [13] reported that pain perception during TRUS-guided prostate biopsy is less profound in patients in the LLD position than in the lithotomy position. Those studies suggested that the lithotomy position may be more unpleasant or embarrassing than the LLD position in terms of psychological phenomenon, because the LLD position is a more relaxed and physiological position typically used for sleep and rest. By using an adjustable stirrup in the operation room, however, we minimized the physical discomfort induced by the lithotomy position during TRUS-guided prostate biopsy. Also, our study was the first to report that the lithotomy position was a significant independent factor related to pain during TRUS-guided prostate biopsy, compared with other factors related to pain, such as PSA, age, and IPSS.
Our study also demonstrated that pain scores during TRUS-guided prostate biopsy are lower in DM patients than in patients without diabetes. Diabetes can damage the peripheral nervous system in various ways [21]. Chronic elevation of blood glucose levels leads to damage to blood vessels and results in diabetic neuropathy [22]. The prevalence of neuropathy in patients with diabetes is about 30% [23]. We hypothesize that pain scores during TRUS-guided prostate biopsy are lower in patients with DM because sensation is significantly decreased in these patients.
Unfortunately, there were several limitations in this series. First, because this study was conducted retrospectively in a single center and included a small number of subjects, we did not investigate other questionnaires regarding anxiety and depression related to prostate biopsy. Therefore, additional prospective studies with larger numbers of patients should be conducted to further evaluate this finding. Second, we did not evaluate pain scores at each step, such as insertion of ultrasound probe and penetration of the needle. Finally, the TRUS-guided prostate biopsy was performed by two different investigators. Nevertheless, to the best of our knowledge, this series was the first report to investigate the effect of the patient's position on pain during TRUS-guided prostate biopsy in Asian men. In addition, it demonstrated that the patient's positioning during TRUS-guided prostate biopsy is a significant independent factor related to pain and that patients in the lithotomy position experienced less pain than did patients in the LLD position.
Patients who underwent TRUS-guided prostate biopsy in the lithotomy position experienced less pain than did those in the LLD position, without a significant difference in the prostate cancer detection rate between the two positions. We suggest that the lithotomy position is the proper way to perform TRUS-guided prostate biopsy to reduce the patient's pain perception, especially in Asian men.
Figures and Tables
Fig. 1
Simple linear regression model of relationship between visual analogue scale (VAS) score and position. LLD, left lateral decubitus. r2=0.051, p=0.011.

Table 1
Characteristics and clinical variables of the patients

Table 2
Pearson correlation coefficient between visual analogue scale score and clinical variables
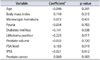
Table 3
Multiple linear regression model of comparison between visual analogue scale score and clinical variables

References
1. Jung KW, Park S, Won YJ, Kong HJ, Lee JY, Seo HG, et al. Prediction of cancer incidence and mortality in Korea, 2012. Cancer Res Treat. 2012; 44:25–31.
2. Elabbady AA, Khedr MM. Extended 12-core prostate biopsy increases both the detection of prostate cancer and the accuracy of Gleason score. Eur Urol. 2006; 49:49–53.
3. Clements R, Aideyan OU, Griffiths GJ, Peeling WB. Side effects and patient acceptability of transrectal biopsy of the prostate. Clin Radiol. 1993; 47:125–126.
4. Collins GN, Lloyd SN, Hehir M, McKelvie GB. Multiple transrectal ultrasound-guided prostatic biopsies: true morbidity and patient acceptance. Br J Urol. 1993; 71:460–463.
5. Scattoni V, Zlotta A, Montironi R, Schulman C, Rigatti P, Montorsi F. Extended and saturation prostatic biopsy in the diagnosis and characterisation of prostate cancer: a critical analysis of the literature. Eur Urol. 2007; 52:1309–1322.
6. Issa MM, Bux S, Chun T, Petros JA, Labadia AJ, Anastasia K, et al. A randomized prospective trial of intrarectal lidocaine for pain control during transrectal prostate biopsy: the Emory University experience. J Urol. 2000; 164:397–399.
7. Szlauer R, Paras L, Fink KG. Addition of lidocaine suppositories to periprostatic nerve block enhances pain control in prostate biopsies: a placebo-controlled randomized trial. Urol Int. 2010; 84:413–417.
8. Tsuji FH, Chambo RC, Agostinho AD, Trindade Filho JC, de Jesus CM. Sedoanalgesia With Midazolam and Fentanyl Citrate Controls Probe Pain During Prostate Biopsy by Transrectal Ultrasound. Korean J Urol. 2014; 55:106–111.
9. Cormio L, Pagliarulo V, Lorusso F, Selvaggio O, Perrone A, Sanguedolce F, et al. Combined perianal-intrarectal (PI) lidocaine-prilocaine (LP) cream and lidocaine-ketorolac gel provide better pain relief than combined PI LP cream and periprostatic nerve block during transrectal prostate biopsy. BJU Int. 2012; 109:1776–1780.
10. Luscombe CJ, Cooke PW. Pain during prostate biopsy. Lancet. 2004; 363:1840–1841.
11. Bruyere F, Faivre d'Arcier B, Haringanji DC, Boutin JM, Haillot O, Lanson Y. Effect of patient position on pain experienced during prostate biopsy. Urol Int. 2007; 78:351–355.
12. Kilciler M, Demir E, Bedir S, Erten K, Kilic C, Peker AF. Pain scores and early complications of transrectal ultrasonography-guided prostate biopsy: effect of patient position. Urol Int. 2007; 79:361–363.
13. Lodeta B, Lodeta M. Prostate biopsy in the left lateral decubitus position is less painful than prostate biopsy in the lithotomy position: a randomized controlled trial. Korean J Urol. 2012; 53:87–91.
14. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014; 65:124–137.
15. Tiong HY, Liew LC, Samuel M, Consigliere D, Esuvaranathan K. A meta-analysis of local anesthesia for transrectal ultrasound-guided biopsy of the prostate. Prostate Cancer Prostatic Dis. 2007; 10:127–136.
16. Turgut AT, Olcucuoglu E, Kosar P, Geyik PO, Kosar U. Complications and limitations related to periprostatic local anesthesia before TRUS-guided prostate biopsy. J Clin Ultrasound. 2008; 36:67–71.
17. von Knobloch R, Weber J, Varga Z, Feiber H, Heidenreich A, Hofmann R. Bilateral fine-needle administered local anaesthetic nerve block for pain control during TRUS-guided multicore prostate biopsy: a prospective randomised trial. Eur Urol. 2002; 41:508–514.
18. Resende AP, Petricelli CD, Zanetti MR, Sanches M, Campanholi V, Nakamura MU, et al. Which of the recumbent positions promoves better pelvic floor muscle relaxation? In : Joint Annual Meeting of the International Continence Society (ICS) and International Urogynecological Association (IUGA); 2010 Aug 23-27; Toronto, Canada.
19. Jadhav SA, Sukumar S, Kumar G, Bhat SH. Prospective analysis of psychological distress in men being investigated for prostate cancer. Indian J Urol. 2010; 26:490–493.
20. Awsare NS, Green JS, Aldwinckle B, Hanbury DC, Boustead GB, McNicholas TA. The measurement of psychological distress in men being investigated for the presence of prostate cancer. Prostate Cancer Prostatic Dis. 2008; 11:384–389.
21. Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012; 11:521–534.
22. Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia. 2000; 43:957–973.
23. Maser RE, Steenkiste AR, Dorman JS, Nielsen VK, Bass EB, Manjoo Q, et al. Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes. 1989; 38:1456–1461.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download