Abstract
Purpose
Prostate cancer is the most frequent cancer in men in Europe. A major focus in urology is the identification of new biomarkers with improved accuracy in patients with low-risk prostate cancer. Here, we evaluated two-dimensional neovascular complexity in prostate tumor and nontumor biopsy cores by use of a computer-aided image analysis system and assessed the correlations between the results and selected clinical and pathological parameters of prostate carcinoma.
Materials and Methods
A total of 280 prostate biopsy sections from a homogeneous series of 70 patients with low-risk prostate cancer (Gleason score 3+3, prostate-specific antigen [PSA]<10 ng/mL, and clinical stage T1c) who underwent systematic biopsy sampling and subsequent radical prostatectomy were analyzed. For each biopsy, 2-µm sections were treated with CD34 antibodies and were digitized by using an image analysis system that automatically estimates the surface fractal dimension.
Results
Our results showed that biopsy sections without cancer were significantly more vascularized than were tumors. No correlations were found between the vascular surface fractal dimension and patient's age, PSA and free-to-total PSA ratios, pathological stage, Gleason score, tumor volume, vascular invasion, capsular penetration, surgical margins, and biochemical recurrence.
Conclusions
The value of angiogenesis in prostate cancer is still controversial. Our findings suggest that low-risk prostate cancer tissues are less vascularized than are nontumor tissues. Further studies are necessary to understand whether angiogenesis is a hallmark of intermediate- and high-risk prostate cancer.
Among men, cancers of the prostate, lung and bronchus, and colorectum account for about 50% of all newly diagnosed cancers [1]. Prostate cancer alone will account for 27% (233,000) of incident cases in men [1]. Although prostate-specific antigen (PSA) is one of the most widely used tumor markers, it has various drawbacks, mainly due to its low specificity and low negative predictive value [2]. Prostate cancer with a Gleason score of 6 (3+3) (GS6) is the most commonly diagnosed prostate cancer among men with cancer detected by PSA screening, the most histologically well differentiated, and the type with the most favorable prognosis [3]. Despite the high prevalence of GS6 cancer, however, considerable debate exists regarding its clinical significance, natural history, metastatic potential, and optimal management [3]. GS6 prostate cancer meets the histologic criteria for adenocarcinoma, although some argue it does not meet the 6 hallmarks of cancer: sustained proliferative signaling, evasion of growth suppressors, resistance of apoptosis, replicative immortality, induction of angiogenesis, and invasion or metastasis [4,5]. Therefore, one of the major focuses in urologic research remains the identification of "quantitative variables" to detect clinically significant GS6 prostate cancers [6,7,8].
Angiogenesis, the development of new branching vessels from existing vasculature, is a complex, dynamic process commonly observed in fetal growth, wound healing, and endometrial hyperplasia [9]. It has been ascertained that tumors are driven by persistently upregulated angiogenesis [9]. Angiogenesis is regulated by a balance of pro- and antiangiogenic molecules secreted from various cells, including cancer cells, endothelial cells, and stromal cells, the relative contributions of which are likely to change with tumor type and site, as well as with tumor growth, regression, and relapse. The implication of angiogenesis in prostate cancer remains debated [10]. While there is currently no accepted biomarker of the angiogenic activity of prostate cancer, it has been ascertained that the measurement of various morphological aspects of tumor vasculature may provide helpful information on angiogenic activity [10,11].
The amount of angiogenesis of prostate cancer is largely estimated by microvessel density (MVD) [12,13]. Although the association between MVD and the survival rate remains controversial, some investigators have proposed MVD as a prognostic and predictive factor [12,14]. MVD has also been associated with tumor aggressiveness, PSA recurrence, and the metastatic potential after radical prostatectomy [15]. MVD has several limitations, however, mainly due to the differences in study design, population size, approach to selection of representative tumor areas, choice of endothelial marker (i.e., factor VIII-related antigen, CD34, CD31, CD105, vascular endothelial growth factor), and measurement method.
The vascular system is a complex ramified network whose geometrical features cannot be analyzed by use of Euclidean geometry, which only interprets regular and smooth objects [10,11]. Fractal geometry, however, is a powerful means of quantifying the spatial complexity of biological systems and their complex behaviors [16,17]. The human vascular system represents a well-characterized, archetypical natural "fractal object" because of its "statistical self-similar" architecture, "noninteger dimension," and "scaling" [18].
Here, we estimated the surface fractal dimension as a "quantitator" of the two-dimensional (2-D) geometrical complexity of microvascularity in 280 prostate biopsy sections by means of a completely automated image analysis procedure (Fig. 1) and assessed correlations between the results and several clinical and pathological parameters of prostate cancer. We investigated the (2-D) irregularly shaped microvascular system in a homogeneous series of low-risk prostate cancer (Gleason score 3+3, PSA<10 ng/mL, and clinical stage T1c) biopsies taken from patients who underwent systematic biopsy sampling and subsequent radical prostatectomy to evaluate whether this feature might be considered a "hallmark of cancer" in this specific group of patients.
A total of 280 prostate biopsies retrospectively taken from 70 patients (1 needle tumor biopsy and 3 nontumor needle biopsies for each patient) who underwent systematic biopsy sampling and subsequent radical prostatectomy for low-risk prostate cancer at the Humanitas Research Hospital (Rozzano, Milan, Italy) from 1999 to 2004 were analyzed. For each patient, serum levels of PSA and the free-to-total (F/T) PSA ratio, clinical and pathological stage, surgical margins, tumor volume, pathological Gleason score, vascular invasion, capsular penetration, and biochemical recurrence were known. All patients enrolled in the study received revised consent forms and were eligible for the study. Biochemical recurrence was defined as a detectable serum PSA level (greater than 0.2 ng/mL) with an increase in this value during 10 years of follow-up. Table 1 shows the demographic, clinical, and pathological characteristics of the patients enrolled in the study.
Two-micrometer-thick sections were cut and processed for immunohistochemistry. After being deparaffinized and rehydrated, the sections were immersed in an antigen retrieval bath (Dako, Milan, Italy) for 30 minutes at 98℃ in 1mM of freshly made ethylenediaminetetraacetic acid solution, incubated with 3% H2O2 for 15 minutes in order to quench endogenous peroxidase activity, and then incubated at room temperature for 2 hours with primary antibodies raised against CD34 (Dako) or 1 mg/mL of mouse IgG1 (Dako) as a negative control. This was followed by a 30-minute incubation with the Envision system (Dako). 3,3'-Diamin obenzidinetetrahydrochloride was used as a chromogen to yield brown reaction products. The nuclei were lightly counterstained with hematoxylin solution.
Tumor specimens were reviewed blind by the same pathologist (P.C.). Gleason score was assigned in the diagnostic evaluation, according to the World Health Organization criteria. None of the specimens harbored tertiary Gleason patterns. Whole biopsy sections were digitized by using a computer-aided image analysis system consisting of a Leica DMLA microscope (Leica, Milan, Italy) equipped with an x-y translator table, a digital camera, and a computer with incorporated ad hoc constructed image analysis software that automatically selected the immunoreactive vascular surface on the basis of RGB (red, green, blue) color segmentation [19]. All of the images were digitized at 10× objective magnification. The 2-D fractal dimension of the immunoreactive vascular surface was estimated by using the box-counting algorithm and the equation, where D is the box-counting fractal dimension of the immunoreactive vascular surface, ε is the side length of the box, and N(ε ) is the smallest number of contiguous and nonoverlapping boxes of side ε required to cover the immunoreactive vascular surface completely [18].
Because the zero limit cannot be applied to biological images, D was estimated by means of the equation where d is the slope of the graph of log N(ε) against log (1/ε). Because natural objects are scale invariant, they maintain their fractal dimension within a fixed range of side lengths (εmin-εmax) based on the constant fitting parameter D [18].
All of the data were expressed as mean values±standard deviation and were analyzed by using Statistica software (StatSoft Inc., Tulsa, OK, USA) and GraphPad Prism 5 (San Diego, CA, USA). Univariate analysis was performed by means of the two-tailed Student t-test or chi-square test for parametric and categorical variables. A p-value of ≤0.05 was considered to be statistically significant.
As shown in Table 1, all patients who underwent systematic biopsy sampling had a diagnosis of low-risk prostate cancer at the time of biopsy with a Gleason score of 3+3, clinical stage T1c, and PSA<10 ng/mL. After radical prostatectomy, the majority of the tumors were graded as 3+3 (44/70 patients, 63%); 26 of 70 patients (37%) had tumors graded 3+4 (Table 2). No significant differences were found in patient's age, PSA and F/T PSA ratio, clinical and pathological stage, tumor volume, capsular penetration, surgical margins, and biochemical recurrence between the two groups. A statistically significant difference was found between the two groups with respect to vascular invasion (p=0.04). Biopsies without cancer had a significantly higher vascular surface fractal dimension than did biopsies with evident prostate cancer (Fig. 2). The same difference was found when the tissues were divided into two groups according to a pathological Gleason score of 3+3 or 3+4 at the subsequent radical prostatectomy (Fig. 2). No correlations were found between the vascular surface and patient's age, PSA and F/T PSA ratio, clinical and pathological stage, Gleason score, tumor volume, vascular invasion, surgical margins, and biochemical relapse.
An increased serum PSA level is the main tool used today to suspect the presence of prostate cancer. Serum PSA quantification has, however, low specificity for detecting prostate cancer and poorly predicts the presence of aggressive disease. A definitive diagnosis of cancer depends on the histopathological analysis of a core needle biopsy sample. In one prospective study, the negative rate of a 12-core biopsy technique was found to be more than 30% [20].
In November 2014, the International Society of Urological Pathology convened a consensus conference on prostate cancer grading in Chicago (IL, USA), and one of the key issues for discussion included the labeling of GS6 prostate cancer and whether it was time to overhaul the terminology used to describe prostate cancer grade [7]. GS6 cancer is the most commonly diagnosed prostate cancer among men with cancer detected by PSA screening, the most histologically well differentiated, and the cancer associated with the most favorable prognosis [3]. Despite the high prevalence of GS6 prostate cancer, however, considerable debate remains regarding its clinical significance, natural history, metastatic potential, and optimal management [3]. GS6 cancer meets the histologic criteria for adenocarcinoma, although some argue it does not meet the six hallmarks of cancer, including induction of angiogenesis.
Angiogenesis is a dynamic process accompanying the development, progression, and metastasis of tumors of unrelated histological origin [9]. Although the role of angiogenesis in prostate cancer remains controversial, several studies have revealed vascular changes in prostate cancer and benign prostatic hyperplasia (BPH) including increased vessels with tiny lumens and irregular shapes, increased vessel density, and reduced blood flow, which suggest that changes in vascular architecture may have a potential role in prostate diseases. Even more controversial is the prognostic value of microvascularity in smaller samples of prostate cancer, such as core needle biopsies [13,21,22]. MVD has not been shown to be a valid measure to guide or evaluate antiangiogenic treatment [23]. In addition, MVD is limited by the highly irregular geometry that the vascular system assumes in real space [18].
Angiogenesis is a nonlinear dynamic process that is discontinuous in space and time, but advances through qualitatively different states. The progression of these states generates a self-similar ramified structure that irregularly fills the surrounding environment. A natural object is said to be self-similar if it can be broken down into arbitrarily small pieces, each of which is a replica of the entire structure [11]. The main feature of the newly generated vasculature is the structural diversity of the vessel sizes, shapes, and connecting patterns. Additionally, it has been ascertained that tumor vessels are structurally and functionally abnormal: unlike normal vessels, they are highly disorganized, tortuous and dilated, and have uneven diameters and excessive branching and shunts [11,18]. The complex geometry of tumor vasculature and its functional heterogeneity mean that microvascularity cannot be measured on the basis of MVD [11,18]. Tretiakova et al. [24] applied automated image analysis to conventional and tissue microarray sections in large representative areas and demonstrated that there was no significant increase in MVD parameters in prostate cancer versus matched normal peripheral zone prostatic tissue. Paradoxically, several morphological indexes were found to be higher within normal glandular prostatic tissue. Deering et al. [25] also reported no increase in MVD counts between BPH and prostate cancer. In 1996, Barth et al. [26] demonstrated that direct stereologic assessment of vascular surface density quantitating the vessel area per tissue volume showed no significant difference between normal and prostate cancer tissue. Another study by Taverna et al. [19] divided the investigated cases in two groups with 56% of cases showing an increase in the vascular surface in prostate cancer versus nontumor areas and 44% showing a decrease in the vascular surface in prostate cancer. The second group of patients with lower tumoral vascular surface had a poorer outcome, which suggests that tumor progression is independent of angiogenesis. These findings parallel recent data that showed no significant difference in CD31 mRNA levels from normal prostate and matched prostate cancer. Additionally, Luczynska et al. [27] found that the lowest MVD was observed in specimens with the highest Gleason scores, tumor grade, and pT classification. It has also been stated that the lack of correlation between vascularity and tumor volume is not surprising. In a higher tumor volume with lower oxygen concentrations, tumor cells can remain viable, and they can exist at greater distances from the vasculature because they acquire the ability to take up glucose and perform glycolysis.
Using a combined approach of immunohistochemistry and computer-aided image analysis, we have quantified the vascular surface fractal dimension in needle biopsy sections with low-risk prostate cancer and matched nontumor biopsy sections and assessed the correlations between these results and selected clinical and pathological parameters of prostate carcinoma. We found that biopsies without cancer had a significantly higher vascular surface fractal dimension than did biopsies showing cancer (Fig. 1, Table 3). In addition, we did not find any correlation between the 2-D vascular complexity and patient's age, serum PSA and the F/T PSA ratio, clinical and pathological stage, tumor volume, vascular invasion, capsular penetration, surgical margins, and biochemical recurrence.
Previously, we demonstrated that vascular surface fractal dimension is a parameter that depends on (1) the number of vessels, (2) the spatial relationships between the vessels, and (3) the interactions between the vascular components and the surrounding tissue [18]. We also developed a computer model capable of generating a large series of 2-D images of a simulated microvascular network [18], and we found that the vascular fractal surface increased with the number of vessels; furthermore, its value changed when the same number of vessels was differently distributed in the surrounding environment [18]. In other words, an equal number of vessels may have different spacefilling properties depending on their distribution pattern. Angiogenesis is a complex process, which involves multiple pathways that are dependent on the homeostatic balance among several growth factors. Although the value of angiogenesis in prostate cancer is still not globally accepted, our findings demonstrate that low-risk prostate cancers are less vascularized than are nontumor tissues.
It is today recognized that prostate cancer is highly heterogeneous in "time" and "space" and that "natural" and "tumoral" prostate microenvironments share some properties, although many other are distinctive and to each state [28,29]. We investigated four biopsy tissues for each patient (i.e., one tumoral and three without prostate cancer). Importantly, the microenvironment of prostate tissue adjacent or distant from the tumor foci cannot be considered absolutely natural, because changes at different levels of observation (i.e., genetic, subcellular entities of cell and tissue as a whole) might not be identifiable at a particular spatial scale [28]. According to our findings, which evaluated a well-recognized "hallmark of cancer," and other reports, GS6 disease should not be labeled with this term [6,30]. However, in our investigated series we found that 1 patient had lymphatic metastasis (pT2bN1), 26 of 70 patients (37%) had a GS of 3+4 at radical prostatectomy, and overall 14 of 70 patients (20%) experienced biochemical recurrence during 10 years of follow-up. These findings encourage all of us to redouble our efforts to improve the comprehension of low-risk prostate cancer.
The results of the present study demonstrate that biopsies taken from men with low-grade prostate cancer have a significantly lower vascular surface fractal dimension than do biopsies without cancer. It has been ascertained that tumors can generate their vasculature in six distinct ways, namely through sprouting angiogenesis, vasculogenesis, intussusception, vessel co-option, vasculogenic mimicry, and transdifferentiation of cancer stem-like cells into tumor endothelial cells. Further studies are necessary to understand whether angiogenesis is a hallmark of intermediate- and high-risk prostate cancer.
Figures and Tables
Fig. 1
(A) The surface fractal dimension, as a numerical index of the two-dimensional geometrical complexity of tumor vascular networks, in patients with prostate cancer. Statistically significant differences were found between the surface fractal dimension in tumor biopsy (TB) samples versus nontumoral biopsy (NTB) cores. The same difference is found when the population is divided in two groups according to the pathological Gleason grade 3+3 or 3+4 at the subsequent radical prostatectomy (B and C, respectively). *p<0.01. ***p<0.0001. ****p<0.00001.

Fig. 2
Multilevel-based procedure for estimating the two-dimensional vascular fractal dimension in tumor and nontumor biopsy cores. It has been recognized that two main issues influence results in the quantification of microvascularity in prostate tissue: (1) subjectivity in the diagnostic interpretation and (2) inadequate quantitative parameter. The present procedure automatically identified the microvascularity and estimated the surface fractal dimension as an index of the geometrical microvascular complexity.
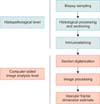
Table 1
Patient population features (n=70)

Table 2
Patients with Gleason score 3+3 and 3+4 at radical prostatectomy
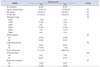
Table 3
Vascular surface fractal dimension in all patients and stratified by Gleason pathological score

ACKNOWLEDGMENTS
The authors are very grateful to Dr. Barbara Franceschini and Sonia Di Biccari for their invaluable technical support.
References
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64:9–29.
2. Stephan C, Ralla B, Jung K. Prostate-specific antigen and other serum and urine markers in prostate cancer. Biochim Biophys Acta. 2014; 1846:99–112.
3. Eggener SE, Badani K, Barocas DA, Barrisford GW, Cheng JS, Chin AI, et al. Gleason 6 prostate cancer: translating biology into population health. J Urol. 2015; 04. 04. [Epub]. http://dx.doi.org/10.1016/j.juro.2015.01.126.
4. Ahmed HU, Arya M, Freeman A, Emberton M. Do low-grade and low-volume prostate cancers bear the hallmarks of malignancy? Lancet Oncol. 2012; 13:e509–e517.
5. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–674.
6. Kulac I, Haffner MC, Yegnasubramanian S, Epstein JI, De Marzo AM. Should Gleason 6 be labeled as cancer? Curr Opin Urol. 2015; 25:238–245.
7. Loeb S, Montorsi F, Catto JW. Future-proofing Gleason grading: what to call Gleason 6 prostate cancer? Eur Urol. 2015; 03. 10. [Epub]. http://dx.doi.org/10.1016/j.eururo.2015.02.038.
8. van der Kwast TH, Roobol MJ. Prostate cancer: Is prostatectomy for Gleason score 6 a treatment failure? Nat Rev Urol. 2015; 12:10–11.
9. Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003; 9:653–660.
10. Taverna G, Grizzi F, Colombo P, Graziotti P. Is angiogenesis a hallmark of prostate cancer? Front Oncol. 2013; 3:15.
11. Grizzi F, Colombo P, Taverna G, Chiriva-Internati M, Cobos E, Graziotti P, et al. Geometry of human vascular system: is it an obstacle for quantifying antiangiogenic therapies? Appl Immunohistochem Mol Morphol. 2007; 15:134–139.
12. Miyata Y, Mitsunari K, Asai A, Takehara K, Mochizuki Y, Sakai H. Pathological significance and prognostic role of microvessel density, evaluated using CD31, CD34, and CD105 in prostate cancer patients after radical prostatectomy with neoadjuvant therapy. Prostate. 2015; 75:84–91.
13. Bostwick DG, Wheeler TM, Blute M, Barrett DM, MacLennan GT, Sebo TJ, et al. Optimized microvessel density analysis improves prediction of cancer stage from prostate needle biopsies. Urology. 1996; 48:47–57.
14. Gettman MT, Bergstralh EJ, Blute M, Zincke H, Bostwick DG. Prediction of patient outcome in pathologic stage T2 adenocarcinoma of the prostate: lack of significance for microvessel density analysis. Urology. 1998; 51:79–85.
15. Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993; 143:401–409.
16. Di Ieva A, Grizzi F, Jelinek H, Pellionisz AJ, Losa GA. Fractals in the neurosciences, part i: general principles and basic neurosciences. Neuroscientist. 2013; 20:403–417.
17. Waliszewski P, Wagenlehner F, Gattenlohner S, Weidner W. Fractal geometry in the objective grading of prostate carcinoma. Urologe A. 2014; 53:1186–1194.
18. Grizzi F, Russo C, Colombo P, Franceschini B, Frezza EE, Cobos E, et al. Quantitative evaluation and modeling of two-dimensional neovascular network complexity: the surface fractal dimension. BMC Cancer. 2005; 5:14.
19. Taverna G, Colombo P, Grizzi F, Franceschini B, Ceva-Grimaldi G, Seveso M, et al. Fractal analysis of two-dimensional vascularity in primary prostate cancer and surrounding nontumoral parenchyma. Pathol Res Pract. 2009; 205:438–444.
20. Chambo RC, Tsuji FH, de Oliveira Lima F, Yamamoto HA, Nobrega de Jesus CM. What is the ideal core number for ultrasound-guided prostate biopsy? Korean J Urol. 2014; 55:725–731.
21. Erbersdobler A, Isbarn H, Dix K, Steiner I, Schlomm T, Mirlacher M, et al. Prognostic value of microvessel density in prostate cancer: a tissue microarray study. World J Urol. 2010; 28:687–692.
22. Taverna G, Grizzi F, Colombo P, Graziotti PP. Microvessel density estimate: friend or foe in the light of prostate vascular system complexity? World J Urol. 2010; 28:405–406.
23. Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn't tell us. J Natl Cancer Inst. 2002; 94:883–893.
24. Tretiakova M, Antic T, Binder D, Kocherginsky M, Liao C, Taxy JB, et al. Microvessel density is not increased in prostate cancer: digital imaging of routine sections and tissue microarrays. Hum Pathol. 2013; 44:495–502.
25. Deering RE, Bigler SA, Brown M, Brawer MK. Microvascularity in benign prostatic hyperplasia. Prostate. 1995; 26:111–115.
26. Barth PJ, Weingartner K, Kohler HH, Bittinger A. Assessment of the vascularization in prostatic carcinoma: a morphometric investigation. Hum Pathol. 1996; 27:1306–1310.
27. Luczynska E, Gasinska A, Wilk W. Microvessel density and expression of vascular endothelial growth factor in clinically localized prostate cancer. Pol J Pathol. 2013; 64:33–38.
28. Grizzi F, Chiriva-Internati M. Cancer: looking for simplicity and finding complexity. Cancer Cell Int. 2006; 6:4.
29. Taverna G, Pedretti E, Di Caro G, Borroni EM, Marchesi F, Grizzi F. Inflammation and prostate cancer: friends or foe? Inflamm Res. 2015; 64:275–286.
30. Lepor H, Donin NM. Gleason 6 prostate cancer: serious malignancy or toothless lion? Oncology (Williston Park). 2014; 28:16–22.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


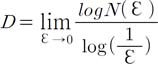
 XML Download
XML Download