Abstract
Purpose
To carry out long-term follow-up of patients diagnosed with asymptomatic simple renal cysts (SRCs).
Materials and Methods
One hundred fifty-eight adult patients in whom SRCs were incidentally diagnosed by abdominal ultrasonography or abdominopelvic computed tomography between August 1994 and June 2004 were followed up for over 10 years. The retrospective analysis investigated sequential changes in the size, shape, and Bosniak classification of the renal cyst and analyzed risk factors for increased size and growth rate of the cysts.
Results
The median follow-up period was 13.9 years (range, 10.0-19.8 years). Median patient age was 54.1 years (range, 22-86 years). Mean maximal cyst size was 33 mm (range, 2-90 mm). Among all patients, 120 (76%) showed a mean increase in maximum renal cyst diameter of 1.4 mm (6.4%) per year. Age at initial diagnosis was a risk factor for increased renal cyst maximum diameter. The probability of an increase in maximum diameter of an SRC was 7.1 times greater in patients aged 50 years or older at diagnosis than in those aged less than 50 years. However, among patients with an increased maximum diameter, the mean growth rate was lower in patients aged ≥50 years than in those aged <50 years.
Conclusions
About three-quarters of adult patients with accidentally diagnosed SRCs presented with an increased maximum diameter. The only risk factor for an increase in maximum diameter was age. In patients with an increase in the maximum diameter, the growth rate of the maximum diameter was 6.4% per year during 10 years and decreased with age.
Simple renal cyst (SRC) is the most common kidney cystic disease. Many are found accidentally in older asymptomatic patients. SRCs are confirmed by diagnostic radiology tests including abdominal ultrasonography or abdominopelvic computed tomography (CT). The prevalence rate of SRCs is about 10% and increases with age [1,2].
The presence of a solitary or multiple renal cysts has generally been considered benign in the absence of a family history of renal cystic disease or evidence of chronic kidney disease. Nonetheless, several recent studies have questioned this consensus by reported associations with the development of hypertension or other symptoms [3,4,5,6,7,8]. For these reasons, some clinicians consider the presence of an SRC to be a disease requiring short-term follow-up. There are no clear guidelines for managing asymptomatic SRCs.
The maximal cyst size of an SRC is thought to be an important factor in the strategy of SRC follow-up. Approximately 2% to 4% of SRCs may become symptomatic (abdominal pain or hematuria) owing to increasing size (6-8 cm) or a complication like infection, hemorrhage, or rupture [6]. Numerous studies have reported that renal cysts can cause hypertension, which frequently resolves after cyst removal [9] or aspiration [10]. One surgical study involving treatment of patients with large renal cysts (mean cyst size, 7.45 cm) suggested a beneficial effect on blood pressure (overall decrease or antihypertensive therapy reduction) in 62% of patients [9].
Despite the availability of studies with long-term follow-up describing the natural history of SRCs in adults, overall consensus on the major risk factors and clinical associations is lacking. This incongruity may be due to variability in the modalities of diagnostic imaging utilized, small cohorts, and combination of data for SRCs and complex renal cysts.
We studied the risk factors for increased renal cyst maximum diameter and the growth rate in patients with SRCs of increasing diameter, with the aim of aiding the treatment strategy for asymptomatic SRCs by retrospective long-term follow-up observations.
Longitudinal changes in asymptomatic SRCs were studied by retrospective investigation of the medical records of 22,349 patients diagnosed with SRCs by abdominal ultrasonography or abdominopelvic CT at a single hospital health clinic from August 1994 to June 2004, with approval from the Institutional Review Board (2015-01-001). Patients with abnormalities of the urinary system, such as renal ectopia or ureteral duplication; patients with other accompanying urinary diseases, such as hydronephrosis or a solid tumor; patients who were being treated for an internal disease, such as hypertension, diabetes, renal failure, or glomerular disease; patients who had a history of treatment for a renal cyst or a family history of polycystic kidney; and patients with abnormalities in serum or on urine tests were excluded. These patients were excluded because of the tendency for early surgical or medical intervention or short-term imaging follow-up, not simply because of an asymptomatic SRC. Urologists consulting on cases of asymptomatic SRCs tried to perform biennial image testing similar to the first exam to the extent that the patients had no new-onset symptoms or signs prompting interventions or changes in the follow-up interval. Thus, 158 patients were included with follow-up observations exceeding 10 years, including at least five radiology tests (Fig. 1).
The diagnosis of SRC was made as follows. The lesion was echolucent on kidney ultrasound, displayed a smooth and clear boundary with a thin but obvious wall, was a round or oval lesion with increased rear echo on ultrasound easily penetrating the cyst, was a round mass with the same concentration of homogenized water on CT, showed no change in shadow concentration in the cyst after the injection of contrast medium, and had a clear lesion boundary with a near cystic parenchyma.
Serial biennial changes including the maximum diameter (Fig. 1), number and shape of the renal cyst (loculation) in each unit, and bilaterality were compared in follow-up observations by use of radiology tests. All serial radiology tests were retrospectively reviewed by one urologist (H.P.). The enrolled patients were divided into two groups: those with an increasing maximum diameter of the SRC and those with no change or a decrease in size of the renal cyst. A change in maximum diameter of within 5% after 10 years compared with the diameter of the initially diagnosed SRC was considered no change owing to measurement error. Factors related to diameter change that were assessed were patient sex and age, cyst size and shape, laterality, number of cysts at the time of diagnosis, and accompanying liver cysts. Age was classified to determine if there was a renal cyst characteristic unique to any age group. Patients showing a change to Bosniak classification class IIF were studied. Additionally, patients with new symptoms owing to a larger cyst underwent laparoscopic cystectomy or intracystic alcohol sclerotherapy.
Normally distributed data were analyzed with Student t-test, with the Mann-Whitney test used for other distributions. Paired t-test was used for normally distributed before and after changes in measured values. Wilcoxon signed rank test was used for other distributions. Frequencies were analyzed with the chi-square test, and the Fisher exact test was used for items with an expectation value exceeding 5. A p-value <0.05 was considered significant. SPSS ver. 10.0 (SPSS Inc., Chicago, IL, USA) was used for the analysis.
The average number and interval of image scans in each patient was 6.4±1.3 (range, 5-9) and 2.6±0.8 years (range, 1.7-4.2 years), respectively. The average number of renal cysts in each unit at the time diagnosis of the 158 enrolled patients was 1.7±1.3, and the mean maximum renal cyst diameter was 32.8±20.0 mm. The total number of renal cysts we evaluated was 320. The median follow-up period was 13.9±2.5 years (range, 10.0-19.8 years). The median age at diagnosis was 54.1±11.0 years (range, 22-86 years). Thirty cases had bilateral renal cysts and 46 cases had more than two renal cysts in a single renal unit. The average number of renal cysts in the latter cases was 3.9±1.8. Seventeen cases were multiloculate renal cysts. Liver cysts were present in 38 patients.
One hundred twenty patients (76%) had an increase in maximum diameter of an SRC at the last follow-up compared with the initial diagnosis. Thirty-eight patients (24%) had no change or a decreased maximum SRC diameter. No significant differences in sex, diagnosed maximum cyst diameter, number of cysts, bilaterality, or cyst shape were observed between the two patient groups. The probability of an increased maximum diameter was 7.1 times greater in patients aged 50 years or older at diagnosis than in patients aged less than 50 years. If the SRC was accompanied by a liver cyst, the likelihood of a maximum diameter increase was 2.1 times greater than in those without a liver cyst. However, whether or not a renal cyst accompanied a liver cyst did not differ in the multivariate analysis (Table 1).
In the group with an increase in maximum diameter, the mean increase in maximum diameter was 1.4 mm (6.43%) per year during the 10-year follow-up. The increase tended to occur more quickly early in the follow-up period and then to stabilize. SRCs of patients aged <50 years displayed more rapid and larger growth to a maximum diameter than did SRCs in patients aged ≥50 years, although the pattern of the growth rate was similar. Most cysts did not grow larger than twice their original size during the 10-year follow-up period (Fig. 2).
Among the 158 enrolled patients, 8 (5.1%) showed a change in renal cyst shape from simple to multiloculated and 2 showed a change in Bosniak class from I to IIF during the follow-up (Fig. 3). For the latter two patients, both of whom were male, one underwent laparoscopic partial nephrectomy with a biopsy result of a SRC. The other was recommended to undergo an operation but refused. Both patients were dropped from follow-up. The renal cyst sizes in the two patients were 70 and 90 mm. The change in the Bosniak classification was found after 11 and 15 years of follow-up, respectively. No factors were found to be related to the changed shape or Bosniak classification of SRCs.
Nine patients (6.3%) among those with initially diagnosed unilateral SRCs developed bilateral SRCs during follow-up. Twelve patients (14%) among those with an initially diagnosed solitary SRC had multiple SRCs during follow-up. There were no factors related to a change in SRC laterality or number.
The increasing use of medical imaging in health clinics has increased the frequency of identification of asymptomatic SRCs in the general population. However, choosing a follow-up strategy and explaining the natural course of SRCs to patient during consultation with a urologist are hindered owing to the absence of standards.
Urological follow-up of patients with asymptomatic SRCs focuses on hypertension and complications. An increasing maximum diameter of an SRC is accepted as an important risk factor for new-onset hypertension and complications during follow-up. Progression to a symptomatic state occurs in 2% to 4% of SRC cases owing to enlargement or the development of a complication, such as hemorrhage, infection, or rupture [6]. In addition, the cysts may cause calyceal or renal pelvic obstruction [11,12,13] and may present with flank pain, abdominal discomfort, a palpable mass, or hematuria. A recent large cross-sectional study conducted in China identified an increasing association between the occurrence and size of SRCs with prehypertension and hypertension [14]. The postulated mechanism of cyst-associated hypertension is renin secretion related to epithelial cells lining the cyst [15]. Increased renin secretion has also been detected in the renal veins of kidneys with very large or perihilar renal cysts [15,16].
A few reports are available on the maximum diameter change of SRCs. One study [17] followed 55 patients (age, 18-79 years) for 3 years among 706 diagnosed with an SRC by ultrasonography and reported increases in maximum diameter of SRCs in 4.2% of patients, with 5.5% of patients showing an increased maximum diameter of an SRC after 6 years. The authors also reported that the maximum diameter of SRCs increased 5% annually, and that the size was 1.6 times larger after 10 years than at the time of diagnosis [17]. Another study followed up SRCs in 61 persons aged 3 to 14 years at 1-year intervals and reported average size increases of 1.6 mm or 3.9% annually [18]. A Japanese study involving 57 adult patients followed up by annual renal ultrasound for a mean duration of 9.9 years detected an average annual increase in cyst size of 1.4 mm and an annual growth rate of 3.2% [5]. Most cysts did not grow larger than twice their original size during the 10-year follow-up. Multivariate analyses determined that age was the most significant determinant of increasing cyst size. The authors also reported that younger patients had a more rapid increase, with the size stabilizing as patients aged [5].
The present study involved 158 patients diagnosed with asymptomatic SRCs by longitudinal investigation who were followed up for over 10 years. Among similar studies, this study had the largest sample size and longest follow-up period. An increase in the maximum diameter of the SRC occurred in 76% of patients, whereas the maximum diameter decreased or did not change in the remaining 24% of patients. The probability of an increase in the maximum diameter of an SRC was higher than in a prior study [17] and lower than in the other aforementioned study [5]. In those with a maximum diameter increase, the mean diameter increase was 6.43% per year during the 10-year follow-up. This result was similar to the findings of Marumo et al. [17] and more rapid than the rate reported by Terada et al. [5]. The latter authors described age as the most significant determinant of an increasing maximum diameter of an SRC in a multivariate analysis. The present study further showed that the probability of a maximum diameter increase of an SRC in patients aged ≥50 years at diagnosis was 7.1 times greater than in patients aged <50 years at diagnosis. As well, among the patients in whom the maximum diameter increased, the mean growth rate of those aged ≥50 years at diagnosis was slower than that in patients aged <50 years.
In the current study, two patients showed a change in Bosniak classification class from I to IIF during the follow-up. The rate of increase in cyst size in these two cases was similar to that in other patients of the same age. Worldwide, only a handful of case reports have described the rare occurrence of SRCs evolving into neoplasms [19,20,21,22]. In all these cases, changes were seen in the characteristics of the cyst wall, emphasizing the essential need for CT imaging to further evaluate any complexity of cysts [12]. Contrastenhanced ultrasound is an emerging technique for assessing complex renal cysts. The technique can provide additional definitive diagnostic information compared to CT, resulting in correct classification of malignant lesions [23].
Despite the longest follow-up period and largest sample size to date, the present study had several limitations. First, this study was conducted retrospectively. To overcome this problem, all serial radiology tests were reviewed by one urologist (H.P.), and only patients who had undergone serial radiology tests at least five times were included. Second, two image modalities were used (abdominal ultrasonography or abdominopelvic CT). However, each patient was tested initially and subsequently by use of the same imaging modality.
About three-quarters of adults with an SRC who were accidentally diagnosed showed an increase in maximum cyst diameter. The only risk factor for the increase was age. In patients with the maximum diameter increase, the diameter growth rate was 6.4% per year during the 10-year follow-up and decreased with age.
Figures and Tables
Fig. 1
Sequential changes in the maximum diameter of the renal cysts in each individual during follow-up. Each maximum diameter is plotted against the patient's age.

Fig. 2
The mean percentile increase in maximum diameter of simple renal cysts during the biennially sequential follow-up period in patients with an increasing maximum diameter.

Fig. 3
Two patients showed a change from Bosniak class I to IIF. (A) In patient 1, a 58-year-old male, cyst size changed from 55 to 70 mm over 11 years. He underwent a laparoscopic partial nephrectomy and the biopsy result was a simple renal cyst. (B) In patient 2, a 90-year-old male, cyst size changed from 73 to 90 mm over 15 years. He refused to undergo an operation (arrow indicates the thickened irregular wall and septa with contrast enhancement).
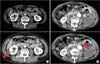
Table 1
Baseline characteristics predicting an increase in maximum cyst diameter

References
1. Tada S, Yamagishi J, Kobayashi H, Hata Y, Kobari T. The incidence of simple renal cyst by computed tomography. Clin Radiol. 1983; 34:437–439.
2. Ravine D, Gibson RN, Donlan J, Sheffield LJ. An ultrasound renal cyst prevalence survey: specificity data for inherited renal cystic diseases. Am J Kidney Dis. 1993; 22:803–807.
3. Carrim ZI, Murchison JT. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol. 2003; 58:626–629.
4. Chang CC, Kuo JY, Chan WL, Chen KK, Chang LS. Prevalence and clinical characteristics of simple renal cyst. J Chin Med Assoc. 2007; 70:486–491.
5. Terada N, Arai Y, Kinukawa N, Terai A. The 10-year natural history of simple renal cysts. Urology. 2008; 71:7–11.
6. Skolarikos A, Laguna MP, de la Rosette JJ. Conservative and radiological management of simple renal cysts: a comprehensive review. BJU Int. 2012; 110:170–178.
7. Hong S, Lim JH, Jeong IG, Choe J, Kim CS, Hong JH. What association exists between hypertension and simple renal cyst in a screened population? J Hum Hypertens. 2013; 27:539–544.
8. Simms RJ, Ong AC. How simple are 'simple renal cysts'? Nephrol Dial Transplant. 2014; 29:Suppl 4. iv106–iv112.
9. Bryniarski P, Kaletka Z, Zyczkowski M, Prokopowicz G, Muskała B, Paradysz A. Ten-year treatment outcomes including blood cell count disturbances in patients with simple renal cysts. Med Sci Monit. 2013; 19:518–523.
10. Zerem E, Imamovic G, Omerovic S. Simple renal cysts and arterial hypertension: does their evacuation decrease the blood pressure? J Hypertens. 2009; 27:2074–2078.
11. Caglioti A, Esposito C, Fuiano G, Buzio C, Postorino M, Rampino T, et al. Prevalence of symptoms in patients with simple renal cysts. BMJ. 1993; 306:430–431.
12. Eknoyan G. A clinical view of simple and complex renal cysts. J Am Soc Nephrol. 2009; 20:1874–1876.
13. Bisceglia M, Galliani CA, Senger C, Stallone C, Sessa A. Renal cystic diseases: a review. Adv Anat Pathol. 2006; 13:26–56.
14. Lee CT, Yang YC, Wu JS, Chang YF, Huang YH, Lu FH, et al. Multiple and large simple renal cysts are associated with prehypertension and hypertension. Kidney Int. 2013; 83:924–930.
15. Solak A, Gur MS, Genc B, Sahin N. Localized cystic disease of the kidney: a rare cause of hypertension in a young adult. J Clin Imaging Sci. 2013; 3:33.
16. Terada N, Arai Y, Kinukawa N, Yoshimura K, Terai A. Risk factors for renal cysts. BJU Int. 2004; 93:1300–1302.
17. Marumo K, Horiguchi Y, Nakagawa K, Oya M, Ohigashi T, Asakura H, et al. Incidence and growth pattern of simple cysts of the kidney in patients with asymptomatic microscopic hematuria. Int J Urol. 2003; 10:63–67.
18. Terada N, Ichioka K, Matsuta Y, Okubo K, Yoshimura K, Arai Y. The natural history of simple renal cysts. J Urol. 2002; 167:21–23.
19. Nishibuchi S, Suzuki Y, Okada K. A case report of renal cell carcinoma in a renal cyst. Hinyokika Kiyo. 1992; 38:181–184.
20. Bowers DL, Ikeguchi EF, Sawczuk IS. Transition from renal cyst to a renal carcinoma detected by ultrasonography. Br J Urol. 1997; 80:495–496.
21. Sakai N, Kanda F, Kondo K, Fukuoka H, Tanaka T. Sonographically detected malignant transformation of a simple renal cyst. Int J Urol. 2001; 8:23–25.
22. Liu JM, Chuang CK, Chang YH, Ng KF, Wang LJ, Chuang KL, et al. A simple renal cyst invaded by infiltrating urothelial carcinoma mimicking a Bosniak Class IV renal cyst. Clin Nephrol. 2011; 76:412–416.
23. Clevert DA, Minaifar N, Weckbach S, Jung EM, Stock K, Reiser M, et al. Multislice computed tomography versus contrast-enhanced ultrasound in evaluation of complex cystic renal masses using the Bosniak classification system. Clin Hemorheol Microcirc. 2008; 39:171–178.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download