Abstract
Purpose
Testicular microlithiasis (TM) is a relatively rare clinical entity of controversial significance characterized by the existence of hydroxyapatite microliths located in the seminiferous tubules. The aim of this study was to observe the natural course of changes in the calcific density of pediatric TM.
Materials and Methods
We included a total of 23 TM patients undergoing scrotal ultrasound (US) on at least two occasions from July 1997 to August 2014. We retrospectively analyzed the patient characteristics, clinical manifestations, specific pathological features, and clinical outcomes. We measured the calcified area and compared the calcific density between the initial and final USs.
Results
The mean age at diagnosis was 11.3±4.6 years, and the follow-up period was 79.1±38.8 months (range, 25.4-152.9 months). During the follow-up period, no patients developed testicular cancer. Calcific density on US was increased in the last versus the initial US, but not to a statistically significant degree (3.74%±6.0% vs. 3.06%±4.38%, respectively, p=0.147). When we defined groups with increased and decreased calcification, we found that diffuse TM was categorized into the increased group to a greater degree than focal TM (10/20 vs. 4/23, respectively, p=0.049). In addition, five of eight cases of cryptorchidism (including two cases of bilateral cryptorchidism) were categorized in the increased calcification group.
The ultrasound (US) appearance of testicular microlithiasis (TM) was first described by Doherty et al. [1] in 1987. These microcalcifications can be identified on US as punctate, nonshadowing, echogenic foci [2]. The prevalence of TM has been reported to be between 2.4%-5.6% [3,4,5]. The prevalence of TM in symptomatic Korean men was found to be 6.0% with significant co-occurrence of TM, testicular cancer, and infertility by Yee et al. [6]
Although the cause-and-effect relationships are unclear, TM has been seen in patients with cryptorchidism, varicoceles, infertility, testicular torsion, Klinefelter syndrome, pulmonary alveolar microlithiasis, neurofibromatosis, acquired immunodeficiency syndrome, intratubular germ cell neoplasia, and most importantly, primary testicular neoplasms [7]. Several authors have also reported the association of TM with testicular cancer. Ikinger et al. [8] reported that 74% of testes with tumors had associated ipsilateral TM on radiological inspection; whereas, only 8% of testicular specimens with benign conditions had microcalcifications. Although Chen et al. [9] reported that there was a significant difference in the rate of malignancy in males with TM compared with those without TM, the question remains whether TM independently increases the risk of testicular malignancy in Taiwanese Men. Ikinger et al. [8], after reporting an association between TM and testicular tumor specimens, suggested in 1982 that radiographic studies be incorporated into diagnosing TM because of the perceived risk for testicular cancer in testicles with microlithiasis.
However, Ganem et al. [10] performed US follow-up in 9 of 22 patients with TM for a mean of 32 months without any newly developing tumors being diagnosed. Bennett et al. [11] and Skyrme et al. [12] reported similar results; therefore, a regular scrotal US is controversial in asymptomatic TM patients. The current recommendations, including those of the European Association of Urology, are that the presence of microlithiasis alone is not an indication for a regular scrotal US in the absence of other risk factors (size<12 mL or atrophy, inhomogeneous parenchyma). TM is not an indication for biopsy or further US screening [13,14,15]. However, there have been no reports describing the changes in calcification over time in pediatric patients with TM. We here report calcific density changes in pediatric TM and their natural course.
The study protocol was approved by the Institutional Review Board of the Asan Medical Center. The medical records from July 1997 to August 2014 of the Asan Medical Center, a tertiary referral center, were retrospectively screened for patients diagnosed with TM by scrotal US. Twenty-three TM patients were included who had undergone scrotal US at least twice. We analyzed the patient characteristics, clinical manifestations, specific pathological features, and clinical outcomes. We measured the calcified area in maximum cross-sectional area, and we compared the calcific density in the initial diagnostic US and the final follow-up US. We defined the testis and the calcified area in terms of their maximal cross-sectional area and calculated those areas using Image J software (National Institutes of Health, Bethesda, MD, USA) (Fig. 1). At diagnosis the maximal cross-sectional area of the testis was divided into nine sections. Patients that showed microlithiasis in three or more sections were defined as diffuse type, and patients with microlithiasis in less than three sections were defined as focal type (Fig. 2). We classified the patients into three groups according to the change of microlithiasis: an increased group, ≥20% increase in microlithiasis; a decreased group, ≥20% decrease in microlithiasis; and a no change group, <20% increase or decrease.
Statistical analyses were performed using the PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). Differences were analyzed by a paired t-test, and crosstabs were used to assess dependent samples. In this study p-values <0.05 were considered statistically significant.
The clinical characteristics of the patients are listed in Table 1. The mean age at presentation was 11.3±4.6 years, and the follow-up period (interval of fist US and last US) was 79.1±38.8 months (range, 25.4-152.9 months). The mean age at last follow-up was 17.8±5.8 years (range, 6.4-26.9 months). Follow-up occurred for 19 of 23 patients over puberty (defined as >13 years old). Of the 23 patients 20 had bilateral TM, 2 patients had unilateral TM, and 1 patient had an atrophic testis. Scrotal US was performed an average of 3.5±1.5 times. We included a total of 43 testes with TM in this study. The most common comorbid condition was cryptorchidism (6 patients, 26.1%). Less frequent comorbid conditions included testicular torsion (3 patients, 13.0%), epididymitis (3 patients, 13.0%), varicocele (2 patients, 8.7%), hydrocele (2 patients, 8.7%), and epididymal cyst (2 patients, 8.7%).
Calcific density was increased at the last follow-up US compared with the initial US; however, this finding was not statistically different (3.74%±6.0% vs. 3.06%±4.38%, respectively, p=0.147). We divided the subjects into two groups (focal vs. diffuse) based on the distribution of TM. We classified 23 testes as having focal TM and 20 testes as having diffuse TM. In focal TM calcific density decreased but not significantly (0.72%±0.55% vs. 0.66%±1.03%, p=0.813). On the other hand, the calcific density of diffuse TM show a trend toward increase (5.8%±5.2% vs. 7.3%±7.4%, p=0.457) (Table 2) (Fig. 3).
To clarify the tendency of TM calcification toward increase vs. decrease we divided the subjects into three groups based on the calcific density change of TM (increase: increased >20%; decrease: decreased >20%; no change: <20% increase or decrease). We categorized 14 testes as increased, 18 testes as decreased, and 11 testes as no change. Half of the patients with diffuse TM were assigned to the increased group, a proportion significantly higher than focal TM (10/20 [50%] in diffuse TM, 4/23 [17.4%] in focal TM, p=0.049) (Table 3). In addition, 5 of 8 testes with cryptorchidism (including 2 with bilateral cryptorchidism) were categorized into the increased group. However, there were no patients who developed testicular cancer or new abnormal symptoms.
Previous TM studies have focused only on the relationship between TM and testicular cancer, and not on the natural course of this disease progression. In our current study, we report the natural course of calcific density changes in pediatric TM during pubertal development. We found that diffuse TM and cryptorchidism are associated with increased calcific density. The mean age at presentation was 11.3 years and the mean follow-up period of approximately 5 years allowed follow-up of 23 patients over pubertal development. Despite a follow-up interval that allowed potential pubertal changes to the testis, none of our patients developed testicular cancer or new abnormal symptoms. By contrast, there are some reported cases of patients with a known TM on US exam that eventually developed a primary testicular cancer. McEniff et al. [16] reported a yolk sac tumor developing in a 17-year-old boy being followed for bilateral TM that was originally detected because of an initial sonographic evaluation of unequal sized testes. Winter et al. [17] reported the case of a man with TM seen on a sonograph performed due to bilateral testicular pain who presented three years later with a metastatic germ cell tumor of the left testicle. Although, an association between TM and subsequent testicular tumors appears likely, whether there is a true cause-and-effect relationship remains unknown. However three other studies-Ganem et al. [10] (9 patients, 32 months), Skyrme et al. [12] (5 patients, 29 months), and Bennett et al. [11] (7 patients, 45 months)-used US to follow patients with TM and, similarly to our present results, did not detect the appearance of new testicular tumors. Compared with these prior studies, our current study incorporates more patients and a longer-term follow-up extending through puberty.
An additional strength of our study over previous studies is in our investigation of changes in calcified density of the TM. One earlier study has reported nonquantitative changes in the prominence of TM on yearly US follow-up. The TM was less prominent in one patient, unchanged in four, and two patients were lost to follow-up [18]. To our knowledge, our present study is the first report to provide a quantitative analysis of calcific density in TM. Calcific density on US showed a nonstatistically significant trend toward increase over time in our study subjects. Bennett et al. [11] reported a relationship between the number of microliths and testicular cancer after subgrouping subjects into four ranges based on the number of microliths. In our present study, we divided TM into two nearly equal groups based on the distribution pattern of calcification (focal type [23 testes] vs. diffuse type [20 testes]) that appear to differ in terms of calcification trends. Calcific density shows a trend toward a decrease in focal TM but toward an increase in 50% of the testes with diffuse TM. Notably, the majority of testes (5/8) in our study series with cryptorchidism, including those with bilateral cryptorchidism, were categorized as being in the increased calcification group.
During the follow-up through puberty of the microlithiasis in the 23 boys in our present study, we observed no significant changes in the density of their microliths and no development of testicular cancer; however, we found that diffuse TM and cryptorchidism tend to increase calcific density. Hence, close observation is recommended in cases of TM combined with cryptorchidism and diffuse microlithiasis.
Figures and Tables
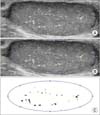 | Fig. 1Measuring method of calcified area. First choose maximum cross-sectional area in ultrasound (A), and define testis area (B) and calcified area by distinct color (C). Finally, calculate testis area and calcified area by image J (National Institutes of Health, Bethesda, MD, USA). Calcific density=calcified area/testis area. |
 | Fig. 2Focal type (A) and diffuse type (B) of testicular microlithiasis. At diagnosis, testis in maximal cross-sectional area was divided in to 9 sites. Patients that showed microlithiasis in 3 or more sites were defined as diffuse and patients with microlithiasis in less than 3 sites were defined as focal. |
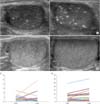 | Fig. 3Change of calcific density demonstrated by the ultrasound image ([A] increase in diffuse type group and [B] decrease in focal type group) and by the bar graph (focal type [C] and diffuse type [D]). |
Table 1
Clinical characteristics of the patients
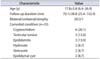
Table 2
Change of calcific density

| First US | Last US | p-value | |
|---|---|---|---|
| All (%) | 3.06±4.38 | 3.74±6.0 | 0.147 |
| Focal TM (%) | 0.72±0.55 | 0.66±1.03 | 0.813 |
| Diffuse TM (%) | 5.8±5.2 | 7.3±7.4 | 0.457 |
References
1. Doherty FJ, Mullins TL, Sant GR, Drinkwater MA, Ucci AA Jr. Testicular microlithiasis. A unique sonographic appearance. J Ultrasound Med. 1987; 6:389–392.
2. Hobarth K, Szabo N, Klingler HC, Kratzik C. Sonographic appearance of testicular microlithiasis. Eur Urol. 1993; 24:251–255.
3. Goede J, Hack WW, van der Voort-Doedens LM, Sijstermans K, Pierik FH. Prevalence of testicular microlithiasis in asymptomatic males 0 to 19 years old. J Urol. 2009; 182:1516–1520.
4. Serter S, Gumus B, Unlu M, Tuncyurek O, Tarhan S, Ayyildiz V, et al. Prevalence of testicular microlithiasis in an asymptomatic population. Scand J Urol Nephrol. 2006; 40:212–214.
5. Hobarth K, Susani M, Szabo N, Kratzik C. Incidence of testicular microlithiasis. Urology. 1992; 40:464–467.
6. Yee WS, Kim YS, Kim SJ, Choi JB, Kim SI, Ahn HS. Testicular microlithiasis: prevalence and clinical significance in a population referred for scrotal ultrasonography. Korean J Urol. 2011; 52:172–177.
7. Rashid HH, Cos LR, Weinberg E, Messing EM. Testicular microlithiasis: a review and its association with testicular cancer. Urol Oncol. 2004; 22:285–289.
8. Ikinger U, Wurster K, Terwey B, Mohring K. Microcalcifications in testicular malignancy: diagnostic tool in occult tumor? Urology. 1982; 19:525–528.
9. Chen JL, Chou YH, Tiu CM, Chiou HJ, Wang HK, Chiou SY, et al. Testicular microlithiasis: analysis of prevalence and associated testicular cancer in Taiwanese men. J Clin Ultrasound. 2010; 38:309–313.
10. Ganem JP, Workman KR, Shaban SF. Testicular microlithiasis is associated with testicular pathology. Urology. 1999; 53:209–213.
11. Bennett HF, Middleton WD, Bullock AD, Teefey SA. Testicular microlithiasis: US follow-up. Radiology. 2001; 218:359–363.
12. Skyrme RJ, Fenn NJ, Jones AR, Bowsher WG. Testicular microlithiasis in a UK population: its incidence, associations and follow-up. BJU Int. 2000; 86:482–485.
13. Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohle G, et al. European Association of Urology guidelines on Male Infertility: the 2012 update. Eur Urol. 2012; 62:324–332.
14. Montgomery JS, Bloom DA. The diagnosis and management of scrotal masses. Med Clin North Am. 2011; 95:235–244.
15. Elzinga-Tinke JE, Sirre ME, Looijenga LH, van Casteren N, Wildhagen MF, Dohle GR. The predictive value of testicular ultrasound abnormalities for carcinoma in situ of the testis in men at risk for testicular cancer. Int J Androl. 2010; 33:597–603.
16. McEniff N, Doherty F, Katz J, Schrager CA, Klauber G. Yolk sac tumor of the testis discovered on a routine annual sonogram in a boy with testicular microlithiasis. AJR Am J Roentgenol. 1995; 164:971–972.
17. Winter TC 3rd, Zunkel DE, Mack LA. Testicular carcinoma in a patient with previously demonstrated testicular microlithiasis. J Urol. 1996; 155:648.
18. Dagash H, Mackinnon EA. Testicular microlithiasis: what does it mean clinically? BJU Int. 2007; 99:157–160.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download