Abstract
Purpose
To investigate the prevalence and clinical significance of incidental prostate fluoro-2-deoxyglucose (FDG) uptake and to evaluate its impact on patient management.
Materials and Methods
Of 47,109 men who underwent FDG positron emission tomography between 2004 and 2014, 1,335 (2.83%) demonstrated incidental FDG uptake in the prostate, with 99 of the latter undergoing prostate biopsy. The primary end point was the histological presence of prostate adenocarcinoma in the biopsy specimen. Outcomes, including treatment methods, survival, and causes of death, were also assessed. Factors associated with the diagnosis of prostate cancer were evaluated by using logistic regression analysis.
Results
Patients with prostate cancer were more likely to have higher serum prostate-specific antigen (PSA) (p=0.001) and focal FDG uptake (p=0.036) than were those without. Prostate cancer occurred in 1 of 26 patients (3.8%) with serum PSA<2.5 ng/mL, compared with 40 of 67 patients (59.7%) with serum PSA≥2.5 ng/mL. Multivariable analysis showed that focal lesions (odds ratio [OR], 5.50; p=0.038), age (OR, 1.06; p=0.031), and serum PSA (OR, 1.28; p=0.001) were independent predictors of prostate cancer diagnosis. Most patients with prostate cancer had organ-confined tumors. Of these, 12 (29.3%) underwent radical prostatectomy and 25 (60.9%) received hormone therapy. Of the 11 patients who died, 9 died of primary cancer progression, with only 1 patient dying from prostate cancer.
Conclusions
The prevalence of incidental FDG uptake in the prostate was not high, although patients with elevated serum PSA had a higher incidence of prostate cancer. Patients with FDG uptake in the prostate should be secondarily evaluated by measuring serum PSA, with those having high serum PSA undergoing prostate biopsy.
Positron emission tomography (PET) is a procedure for evaluating biochemical and physiological processes by use of radiopharmaceuticals labeled with positron-emitting radionuclides. 18F-fluoro-2-deoxyglucose (18F-FDG) PET scanning has been widely utilized for the diagnosis of various tumors; for the staging and restaging of various malignancies, including head and neck, esophageal, lung, breast, colorectal, and gynecological cancers, as well as melanoma and lymphoma [1,2]; and for assessing brain function and heart muscle metabolism [3]. However, the positive predictive value of 18F-FDG PET is low for cancer [4,5], including for prostate cancer [6].
FDG uptake in the prostate is nonspecific for cancer and may result from an inflammatory condition such as prostatitis [7]. Prostate cancer shows no or mild FDG uptake because of its low glucose metabolism [8]. Moreover, the maximum standardized uptake value (SUV) of FDG is not essential for the differential diagnosis of prostatic lesions [9]; nevertheless, several studies have reported a high correlation between FDG uptake and prostate cancer [1,6,9]. For example, FDG uptake without coincidental calcification indicates the possibility of prostate cancer, suggesting the need for additional diagnostic methods, including digital rectal examinations (DREs) and measurements of serum concentrations of prostate-specific antigen (PSA) [3,5].
Incidental FDG uptake in the prostate is often experienced in clinical practice, but SUV alone cannot determine whether increased uptake indicates a malignancy or a benign state. Detection of second primary cancers, particularly early cancers that require radical treatment, is important because these cancers can significantly influence patient management [10,11]. We therefore investigated the prevalence and clinical significance of incidental prostate FDG uptake and evaluated its impact on patient management.
The medical records of all patients who underwent FDG PET at our hospital between October 2004 and March 2014 were reviewed. The study was performed with the approval and oversight of the Institutional Review Board, which waived the requirement for informed consent because of the retrospective design of this study.
During the study period, 92,203 patients (47,109 males and 45,094 females) underwent FDG PET. Of the 47,109 males, 1,335 (2.83%) demonstrated incidental FDG uptake in the prostate. Of the 144 patients who underwent prostate biopsy, the 45 who had undergone FDG PET for staging or restaging of prostate cancer were excluded. Finally, 99 patients who underwent prostate biopsy following FDG uptake in the prostate were included in this study (Fig. 1).
Patients underwent FDG PET on one of the three scanners operated in our hospital (Discovery ST [GE Healthcare, Wauwatosa, WI, USA] and Biograph 16 and Biograph 40 [Siemens Medical Solutions, Malvern, PA, USA]). All patients fasted for at least 6 hours before the examination. After their venous blood glucose concentration was confirmed as being <150 mg/dL, the patients were intravenously injected with 18F-FDG (5.2 MBq/kg body weight), with PET/computed tomography (CT) scanning started 50 minutes later. Images were reconstructed by using a three-dimensional ordered subset expectation maximization algorithm, and CT attenuation maps were used for attenuation correction. PET data were acquired immediately from the same body region. PET, CT, and fused PET/CT images were available for review and were displayed in axial, coronal, and sagittal planes on a viewer system. The SUV was calculated according to the standard formula, with the use of lean body mass as the body weight. FDG uptake in the prostate was visually defined as positive or negative, with physiological uptake in the prostate urethra on coronal, sagittal, and axial views considered negative. The maximum SUV for the prostate was obtained from transaxial views. Patterns of FDG uptake (focal or diffuse) were evaluated on axial views (Fig. 2).
Baseline demographic and clinical characteristics, including patient age, serum total PSA concentration, DRE findings, and transrectal ultrasound (TRUS)-derived information (prostate volume) were obtained by reviewing medical records. PSA concentrations were measured by using a PSA-RIACT assay system (CIS Bio International, Gif-Sur-Yvette, France). Prostate volume was calculated by TRUS by using the formula: volume=(π/6)×length×width×he ight. All patients underwent TRUS-guided laterally directed systematic 12-core biopsies of the prostate
The primary study end point was the histological presence of adenocarcinoma of the prostate in the biopsy specimen. All samples were graded by genitourinary pathologists at our institute, with grading based on the Gleason scoring system. The outcomes in patients with prostate cancer, including treatment methods, survival, and causes of death, were also assessed. Patients were divided into three groups according to their prostate biopsy results, as follows: those with normal prostate, prostate cancer, and prostate invasion, except for prostate cancer.
Clinicopathological factors were compared in the benign lesion and prostate cancer groups by using Pearson chisquare test for categorical variables and Student t-test for continuous variables. Quantitative data are expressed as mean plus standard deviation or as median and range. Factors associated with a diagnosis of prostate cancer were evaluated by using logistic regression analysis, with variables of p<0.1 on univariate analysis included in the multivariable analysis. Correlations between clinical outcomes and the assessed variables are expressed as odds ratios (ORs) and 95% confidence intervals (CIs). The group of patients with prostate invasion, except for prostate cancer, was analyzed separately. All statistical tests were two-sided, with p<0.05 considered statistically significant. All statistical analyses were performed by using the PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Table 1 shows the clinical characteristics of the 52 patients with benign lesions and the 41 patients (44.1%) with prostate cancer. The mean age of all 93 patients was 65.8 years, with the mean ages of the benign lesion and prostate cancer groups being 62.8 and 69.6 years, respectively (p=0.001). Patients with prostate cancer were more likely to have higher serum PSA concentrations (p=0.001) and focal FDG uptake (p=0.036). Only 1 of the 26 patients (3.8%) with serum PSA<2.5 ng/mL had prostate cancer, compared with 40 of the 67 patients (59.7%) with serum PSA≥2.5 ng/mL. The TRUS-determined prostate volume in all 93 patients was 38.8 mL and was similar in the benign lesion and prostate cancer groups (39.7 mL vs. 37.7 mL, p=0.672). In addition, mean maximum SUV was similar in the two groups (p=0.116).
Univariate analysis showed that age (OR, 1.08; 95% CI, 1.02-1.13; p=0.003), serum PSA concentration (OR. 1.31; 95% CI, 1.14-1.50; p=0.001), and the presence of focal lesions (OR, 3.80; 95% CI, 1.10-14.50; p=0.043) were significant predictors of a final diagnosis of prostate cancer. Multivariable analysis showed that the presence of focal lesions was an independent predictor of a diagnosis of prostate cancer (OR, 5.50; 95% CI, 1.09-27.64; p=0.038), as were age (OR, 1.06; 95% CI, 1.01-1.11; p=0.031) and serum PSA concentration (OR, 1.28; 95% CI, 1.10-1.47; p=0.001) (Table 2).
Table 3 shows the clinical characteristics of the six patients with prostate invasion, except for prostate cancer. These six patients had a mean age of 59.0 years, a mean serum PSA concentration of 3.2 ng/mL, and a mean prostate volume of 49.3 mL. Histologically, two of these patients (33.3%) had bacillus Calmette-Guerin-granuloma, two (33.3%) had lymphoma, one (16.7%) had gastrointestinal stromal tumor, and one (16.7%) had colon cancer.
Table 4 shows the patterns of treatment in the 41 patients with prostate cancer, most of whom had organconfined tumors. Of these 41 patients, 12 (29.3%) underwent radical prostatectomy and 25 (60.9%) received hormone therapy. Of the 11 patients who died, 9 did so from primary cancer progression, with only 1 patient dying from prostate cancer.
Because 18F-FDG PET is becoming more widely used in cancer diagnosis, staging, and restaging, as well as in monitoring response to treatment, more incidental lesions, including prostate lesions, are being detected. The rise in incidental findings has created a problem for clinicians and often leads to unnecessary diagnosis and treatment, consequently increasing patient concern. Thus, determining the clinical significance of FDG uptake in the prostate is extremely important. Our results showed that prostate cancer was strongly associated with serum PSA concentration, with tumors detected in 40 of 67 patients (59.7%) with serum PSA≥2.5 ng/mL but in only 1 of 26 patients (3.8%) with serum PSA<2.5 ng/mL. This difference is much greater than that observed in community-based populations. In general, prostate cancers are detected in approximately 2% of men with PSA of 2-4 ng/mL and in 11% of those with PSA of 4-10 ng/mL [12,13]. Our multivariate analysis also showed that the serum PSA concentration was an independent predictor of a diagnosis of prostate cancer (OR, 1.28; 95% CI, 1.10-1.47; p=0.001). These findings suggest that patients with FDG uptake in the prostate should undergo secondary evaluation, including measurement of serum PSA, and that those with high serum PSA should be evaluated by prostate biopsy.
FDG PET is relatively insensitive in the detection of primary tumors, with sensitivities ranging from 19% to 64% [14]. FDG uptake in the prostate is not specific to cancer, but is also positive in patients with benign prostatic hypertrophy (BPH) and inflammatory conditions such as prostatitis [15]. FDG uptake tends to be higher in poorly differentiated prostate tumors and in those with higher serum PSA concentrations than in tumors with lower PSA levels, a more localized clinical stage, and lower Gleason scores [16,17], perhaps because the level of glucose transporter-1 expression rises with increasing malignancy grade [18]. Therefore, FDG PET may not be useful in the diagnosis or staging of clinically organ-confined disease, because the levels of FDG accumulation in normal prostate tissue, BPH, and prostate cancer may overlap [14,15]. However, focal FDG uptake is important because most of our patients with prostate cancer had focal FDG uptake, and other studies have reported that focal uptake in the peripheral zone correlates with prostate cancer [5,6,19]. BPH is characterized by nodular hyperplasia of the fibromuscular tissue and epithelium within the transition zone and periurethral area [20]. By contrast, most prostate cancers arise from the peripheral zone [21].
In some solitary tumors, maximum SUV closely correlates with pathologic grade, which suggests that this parameter could be used for diagnosis and for assessing prognosis [22]. In our study and others, however, there was no correlation between the maximum SUV of uptake in the prostate and prostate cancer [6,19]. Although the degree of FDG uptake by primary prostate tumors was greater in patients with higher PSA than in those with lower PSA levels, PSA levels can also increase in benign conditions such as BPH [23,24].
Radical prostatectomy is the treatment of choice for organ-confined prostate cancer [25]. An ideal candidate for radical prostatectomy is free of comorbidities that may make the operation unacceptably risky. Surgery is not indicated for patients with a large number of comorbidities or a short life expectancy, however, and these patients should receive hormone therapy or radiation therapy, which may effectively control tumor progression [25,26]. Of our 41 patients with prostate cancer, 37 (90.2%) received treatment, with only 1 patient (2.4%) dying of prostate cancer; thus, a diagnosis of prostate cancer in patients with incidental prostate FDG uptake could have affected their prognosis.
This study had several limitations, including its retrospective design, the relatively small patient cohort, and the significant differences in several clinical variables between the groups of patients with normal prostate and prostate cancer. Moreover, our study was limited by selection bias, because prostate cancer was not histologically confirmed in all patients. Although the most accurate tool for diagnosing prostate cancer is biopsy, some patients showing incidental focal FDG uptake on PET did not undergo prostate biopsy; thus, the sensitivity of FDG PET could not be determined in this patient cohort. Nonetheless, the present study is the first to analyze the clinical significance of incidental prostate FDG uptake and to evaluate the prevalence of prostate cancer according to PSA and clinical outcomes. This study showed that patients with FDG uptake and elevated serum PSA should be evaluated by prostate biopsy, especially if their scans show focal lesions.
The prevalence of incidental FDG uptake in the prostate was not high; however, patients with elevated serum PSA had a high incidence of prostate cancer. Therefore, patients showing FDG uptake in the prostate should undergo further evaluation, including measurements of serum PSA concentration, with those having a high serum PSA evaluated by prostate biopsy. The results of this study may be helpful in planning the management of patients with incidental FDG uptake in the prostate. Because the study cohort was relatively small, however, further studies are needed to determine the clinical validity of this management strategy.
Figures and Tables
 | Fig. 1Flow diagram of patient selection. 18F-FDG PET, 18F-fluoro-2-deoxyglucose positron emission tomography; w/u, workup. |
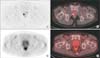 | Fig. 2Axial views of FDG uptake by the prostate. (A) Diffusion-weighted image showing focal uptake. (B) PET image showing focal uptake (maximum SUV, 13.3). (C) Diffusion showing diffuse uptake. (D) PET image showing diffuse uptake (maximum SUV, 3.1). FDG, fluoro-2-deoxyglucose; PET, positron emission tomography; SUV, standardized uptake value. |
Table 1
Demographic and clinical characteristics of patients with normal prostate and prostate cancer, as determined by biopsy results

Table 2
Factors associated with a diagnosis of prostate cancer

Table 3
Demographic and clinical characteristics of the six patients with prostate invasion, except for prostate cancer

Table 4
Patterns of treatment of patients with prostate cancer

ACKNOWLEDGMENTS
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI06C0868).
References
1. Han EJ, O JH, Choi WH, Yoo IR, Chung SK. Significance of incidental focal uptake in prostate on 18-fluoro-2-deoxyglucose positron emission tomography CT images. Br J Radiol. 2010; 83:915–920.
2. Facey K, Bradbury I, Laking G, Payne E. Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess. 2007; 11:iii–iv. xi–267.
3. Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002; 2:683–693.
4. Schaaf WE Jr, Patel Z, Retrouvey M, Cunningham TD, Johnson LS. Frequency and clinical relevance of PET/CT incidentalomas. Abdom Imaging. 2014; 39:657–662.
5. Nayan S, Ramakrishna J, Gupta MK. The proportion of malignancy in incidental thyroid lesions on 18-FDG PET study: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2014; 151:190–200.
6. Hwang I, Chong A, Jung SI, Hwang EC, Kim SO, Kang TW, et al. Is further evaluation needed for incidental focal uptake in the prostate in 18-fluoro-2-deoxyglucose positron emission tomography-computed tomography images? Ann Nucl Med. 2013; 27:140–145.
7. Kao PF, Chou YH, Lai CW. Diffuse FDG uptake in acute prostatitis. Clin Nucl Med. 2008; 33:308–310.
8. Takahashi N, Inoue T, Lee J, Yamaguchi T, Shizukuishi K. The roles of PET and PET/CT in the diagnosis and management of prostate cancer. Oncology. 2007; 72:226–233.
9. Hyun SH, Choi JY, Lee KH, Choe YS, Kim BT. Incidental focal 18F-FDG uptake in the pituitary gland: clinical significance and differential diagnostic criteria. J Nucl Med. 2011; 52:547–550.
10. Leon X, Ferlito A, Myer CM 3rd, Saffiotti U, Shaha AR, Bradley PJ, et al. Second primary tumors in head and neck cancer patients. Acta Otolaryngol. 2002; 122:765–778.
11. Lim JH, You D, Jeong IG, Hong JH, Ahn H, Kim CS. Prognosis of prostate cancer with other primary malignancies. Korean J Urol. 2014; 55:327–334.
12. Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004; 350:2239–2246.
13. Oesterling JE, Jacobsen SJ, Chute CG, Guess HA, Girman CJ, Panser LA, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of agespecific reference ranges. JAMA. 1993; 270:860–864.
14. Kumar R, Zhuang H, Alavi A. PET in the management of urologic malignancies. Radiol Clin North Am. 2004; 42:1141–1153.
15. Liu IJ, Zafar MB, Lai YH, Segall GM, Terris MK. Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology. 2001; 57:108–111.
16. Oyama N, Akino H, Suzuki Y, Kanamaru H, Sadato N, Yonekura Y, et al. The increased accumulation of [18F]fluorodeoxyglucose in untreated prostate cancer. Jpn J Clin Oncol. 1999; 29:623–629.
17. Dong A, Bai Y, Wang Y, Zuo C, Lu J. Spectrum of the prostate lesions with increased FDG uptake on (18)F-FDG PET/CT. Abdom Imaging. 2014; 39:908–921.
18. Effert P, Beniers AJ, Tamimi Y, Handt S, Jakse G. Expression of glucose transporter 1 (Glut-1) in cell lines and clinical specimens from human prostate adenocarcinoma. Anticancer Res. 2004; 24(5A):3057–3063.
19. Yang Z, Hu S, Cheng J, Xu J, Shi W, Zhu B, et al. Prevalence and risk of cancer of incidental uptake in prostate identified by fluorine-18 fluorodeoxyglucose positron emission tomography/ computed tomography. Clin Imaging. 2014; 38:470–474.
20. Untergasser G, Madersbacher S, Berger P. Benign prostatic hyperplasia: age-related tissue-remodeling. Exp Gerontol. 2005; 40:121–128.
21. McNeal JE. The zonal anatomy of the prostate. Prostate. 1981; 2:35–49.
22. Lee HY, Jeong JY, Lee KS, Kim HJ, Han J, Kim BT, et al. Solitary pulmonary nodular lung adenocarcinoma: correlation of histopathologic scoring and patient survival with imaging biomarkers. Radiology. 2012; 264:884–893.
23. Oyama N, Akino H, Kanamaru H, Okada K. Fluorodeoxyglucose positron emission tomography in diagnosis of untreated prostate cancer. Nihon Rinsho. 1998; 56:2052–2055.
24. Reynolds MA, Kastury K, Groskopf J, Schalken JA, Rittenhouse H. Molecular markers for prostate cancer. Cancer Lett. 2007; 249:5–13.
25. Kazzazi A, Momtahen S, Bruhn A, Hemani M, Ramaswamy K, Djavan B. New findings in localized and advanced prostate cancer: AUA 2011 review. Can J Urol. 2011; 18:5683–5688.
26. Carter HB. American Urological Association (AUA) guideline on prostate cancer detection: process and rationale. BJU Int. 2013; 112:543–547.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download