Abstract
Purpose
To evaluate the efficacy and safety of holmium laser enucleation of the prostate (HoLEP) for extremely large prostates.
Materials and Methods
Patients undergoing HoLEP between July 2008 and December 2013 from the Seoul National University Hospital Benign Prostatic Hyperplasia Database Registry were retrospectively analyzed. The patients were divided into three groups according to their total prostate volume (TPV): group A (TPV<100 mL), group B (100 mL≤TPV<200 mL), and group C (TPV≥200 mL); the clinical data of the three groups were compared. All patients were followed up 2 weeks, 3 months, and 6 months after surgery.
Results
A total of 502 patients (group A, 426; group B, 70; group C, 6) with a mean age of 69.0 (standard deviation, ±7.3) years were included in our analysis. The mean prostate volume and prostate-specific antigen level were 68.7±36.9 mL and 4.15±4.24 ng/mL, respectively. The enucleation and morcellation times were longer in group C (p<0.001), and the enucleation efficacy was higher in this group (p<0.001, R2=0.399). Moreover, the mean postoperative catheterization and hospitalization periods were significantly longer in group C (p=0.004 and p=0.011, respectively). However, there were no significant differences between the groups in any other postoperative events, including recatheterization, reoperation, urinary tract infection, clot retention, and bladder neck contracture (p range, 0.516-0.913). One patient in group C experienced recurrence of the urethral stricture.
Holmium laser enucleation of the prostate (HoLEP) is a minimally invasive procedure for lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH) and is independent of prostate size [1,2]. Because HoLEP can be quite challenging, its learning curve is steeper than that of other LUTS/BPH surgical techniques [3,4,5]. However, HoLEP has demonstrated treatment outcomes comparable to those of open prostatectomy and transurethral prostatectomy (TURP). Moreover, compared with TURP, HoLEP is associated with a lower rate of perioperative complications and a shorter time of urethral catheter indwelling [6,7,8,9,10,11]. Therefore, in recent years, this procedure has been increasingly adopted for the treatment of LUTS/BPH.
There are some previous reports on the safety and efficacy of HoLEP for the treatment of large prostates up to 200 mL in size, with HoLEP use reportedly being feasible for large prostates [12,13,14]. Moreover, HoLEP has been shown to have a higher enucleation efficacy and to be associated with a higher enucleated tissue weight per enucleation time in large prostates [4]. However, to date, there have been no reports regarding the safety and efficacy of HoLEP in extremely large prostates (≥200 mL). Thus, the aim of this study was to assess the efficacy and safety of HoLEP in extremely large prostates by retrospectively analyzing patients treated by HoLEP at our institution.
The protocol for the current study was approved by the Institutional Review Board of Seoul National University Hospital, Seoul, Korea (protocol No. H-1404-116-575). Patients who underwent HoLEP for LUTS/BPH between July 2008 and December 2013 were included in this study. Patients with a history of genitourinary surgery or radiation, urinary tract infection, interstitial cystitis, genitourinary malignancy, or neurogenic bladder or who were missing preoperative transrectal ultrasonography (TRUS) evaluation were excluded from the study. The patients were divided into three groups according to the total prostate volume (TPV) on TRUS: group A (TPV<100 mL), group B (100 mL≤TPV<200 mL), and group C (TPV≥200 mL).
All operations were performed by a single surgeon (S.J.O.). The detailed procedure has been previously described [15]. Briefly, the surgical challenges in extremely large prostates are as follows: First, insufficient cystoscopic length is commonly encountered in the enucleation step, which makes it difficult to ensure a clear cystoscopic view beyond the external sphincter, verumontanum, and bladder neck (Fig. 1B; Video clip 1, Supplementary material). Second, identification of the bilateral ureteral orifices is sometimes difficult owing to their acute angle (Fig. 1A); therefore, there is a risk of ureteral orifice injury while making incisions at the 5- and 7-o'clock positions at the mucosal side of the bladder neck (Fig. 1B; Video clip 2, Supplementary material). Third, the cystoscopic view is very restricted, and a risk for bladder mucosal injury exists, owing to the short distance to the bladder wall (Fig. 1B; Video clip 3, Supplementary material). Fourth, the enlarged adenomas restrict the manipulation of the cystoscope (Fig. 1C). Fifth, in larger prostates, more adenoma nodules generally exist. In those cases, it is hard to determine the preferred capsule plane (Fig. 1C; Video clip 4, Supplementary material). Sixth, large prostates tend to have more abundant vessels; therefore, unclear cystoscopic visions owing to bleeding occur more frequently (Video clip 5, Supplementary material). Lastly, upon completion of the enucleation, pushing the enucleated tissue into the bladder for morcellation is often difficult owing to entrapment of adenoma tissue at the narrow bladder neck (Fig. 1D). In the morcellation step, the relatively small bladder space compared to the large prostate adenoma increases the risk of bladder mucosal injury. Therefore, the disturbed cystoscopic view makes it hard to distinguish the adenoma tissue from the normal bladder mucosa. Moreover, the frequency of hard nodules increases with increasing prostate size, and they are observed as round-shaped hard adenoma tissue. Occasionally, these hard nodules require adjuvant transurethral resection (TUR) for retrieval out of the bladder (Video clip 6, Supplementary material).
All patients were routinely followed up at 2 weeks, 3 months, and 6 months after surgery. The preoperative and perioperative parameters, complications, and treatment outcomes at 6 months after surgery were compared. The enucleation ratio was calculated as the enucleated tissue weight per transition zone volume. The enucleation and morcellation efficacies were calculated as the enucleated tissue weight per enucleation time and morcellated tissue weight per morcellation time, respectively.
The comparisons were performed by using the Kruskal- Wallis test for continuous variables and the chi-square test for categorical variables, followed by post hoc analysis between the compared parameters. For comparison of pre- and postoperative parameters, the paired t-test was utilized. All comparisons were two-tailed, and p-values <0.05 were considered significant. Statistical analysis was performed by using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA).
A total of 574 patients were included in the current study. According to the exclusion criteria, 72 patients were excluded from the analysis, resulting in 502 patients being retrospectively analyzed. The patients' mean age was 69.0 (standard deviation, ±7.3) years, and their mean prostate volume and level of prostate-specific antigen were 68.7±36.9 mL and 4.15±4.24 ng/mL, respectively.
Of all 502 patients, 426 (84.9%), 70 (13.9%), and 6 patients (1.2%) were classified into groups A, B, and C, respectively (Table 1). The patient age was similar in all groups (p=0.424); however, a history of hypertension was more common in group C than in the other groups (p=0.009). The quality of life item of the International Prostate Symptom Score (IPSS) differed significantly between groups B and C (p=0.017). In uroflowmetry, the post-voided residual (PVR) volume differed significantly between groups A and B (p=0.005). In the preoperative urodynamic study, group C showed a higher bladder outlet obstruction index and detrusor pressure at maximum flow rate than did the other groups (p=0.006 and p=0.004, respectively) (Table 1).
The mean enucleation time of all subjects was 40.9±19.6 minutes. The mean weight of the enucleated tissue was 24.2±23.4 g, and the mean enucleation ratio was 0.54. The mean enucleation and morcellation efficacies were 0.55±0.35 g/min and 2.07±1.17 g/min, respectively.
The enucleation and morcellation times were significantly longer in group C (p<0.001), and the enucleation efficacy was higher in this group (p<0.001, R2=0.399) (Table 2, Fig. 2). More frequent adjuvant TUR for morcellation or adjuvant transurethral coagulation was necessary for larger prostates. There was no major bleeding requiring transfusion in any group. In one patient in group C, an enucleated adenoma was retrieved via a suprapubic incision instead of via morcellation.
The mean postoperative catheterization and hospitalization periods were longer in group C (p=0.004 and p=0.011, respectively). However, there were no significant differences in any other postoperative events, including recatheterization, reoperation, urinary tract infection, clot retention, and bladder neck contracture (p range, 0.516-0.913). One patient in group C, who had preoperative urethral stricture, showed urethral stricture recurrence after HoLEP, but did not require further surgical intervention (p<0.001). Moreover, transient postoperative incontinence was more frequent in group C than in the other two groups (p=0.015). The rate of adjuvant anticholinergic medication did not differ significantly among the groups (p=0.060). At 6 months after surgery, both groups A and B demonstrated significant improvement of the total sum and quality of life items of the IPSS, maximum flow rate, and PVR volume (Table 3). Group C showed a similar tendency, except for the PVR (p=0.426).
HoLEP for prostates larger than 200 mL has been reported by some groups [6,16]. However, no consensus currently exists about the upper limit of prostate size for the indication of HoLEP.
In our series, the HoLEP procedures in all patients in group C were performed after the surgeon had acquired 180 cases of experience. All HoLEP procedures in group C were challenging procedures, even after the surgeon had overcome the learning curve, which required approximately 50 cases. Therefore, the enucleation time was significantly longer in group C than in the other two groups. However, the enucleation efficacy of group C was also significantly higher than in the other groups (Table 2, Fig. 2), which suggests that HoLEP, unlike other LUTS/BPH surgeries, does not show a linear correlation between operation time and prostate size. This implies that the enucleation time is related not to the volume but rather to the surface area of the prostate. These results are consistent with those of a previous study, which reported that HoLEP is a size-independent procedure [4].
In the current study, the enucleation ratio of group C was 0.63, which was similar to that of the other two groups (p=0.984 and p=0.330, by post hoc analysis) (Table 2). The enucleated tissue weight in this study tended to be less than that of transition zone volume on TRUS, likely owing to the vaporization caused by the laser. These findings were similar to those from other studies [1] and our previous study [17]. Hence, we believe that complete removal of adenoma tissue in extremely large prostates is feasible.
The short-term treatment outcomes of group C improved in terms of the IPSS and uroflowmetry parameters except for the PVR (Table 3). This is consistent with the short-term results of previous studies of HoLEP in smallor moderate-sized prostates, which reported significant improvements of IPSS and uroflowmetric parameters [8,17,18]. The discordance of PVR is likely owing to the small sample size (n=6) in the present study. To confirm these results, a large-scale study of patients with extremely large prostates treated with HoLEP is needed.
In our six cases, no enucleation failures requiring conversion to TURP or open prostatectomy occurred. However, owing to the existence of hard nodules in the morcellation step, adjuvant TURP was necessary in two cases (33.3%). Moreover, in one patient with heart problems, open retrieval of the enucleated adenoma was performed via a suprapubic incision to shorten the anesthetic time. After morcellation, two patients (33.3%) received adjuvant transurethral coagulation for minor bleeding control.
The mean age of the patients in group C was 72.7 years, and all patients had comorbidity. Moreover, two patients (33.3%) were receiving anticoagulants. After a 1-week suspension of the anticoagulants, all patients safely underwent HoLEP. The safety of HoLEP in patients receiving anticoagulants has been previously reported [19]. Our experience suggests that HoLEP can be performed safely with anticoagulants in patients with extremely large prostates.
The mean duration of catheterization in group C was 4.8 days; this was significantly longer than in groups A and B (p=0.025 and p=0.004, respectively; post hoc analysis) (Table 2). However, penile urethral stricture was detected in one patient preoperatively, and endoscopic internal urethrotomy was hence performed prior to HoLEP. In that case, a urethral catheter was indwelled for 13 days. Excluding this case, the mean catheterization duration was 2.8±2.7 days, which is longer than that of a previous study of HoLEP in relatively large prostates (mean volume, 89-114 g; duration, 1.3-1.5 days). However, this is still shorter than the catheterization time for open prostatectomy (duration, 4.1-8.1 days) [10,20]. Recatheterization, reoperation, or readmission within 6 months after surgery was not required in any patient. Transient urge incontinence developed in two patients (33.3%), who improved after anticholinergic treatment. Thus, our early experiences suggest that HoLEP can be performed safely in extremely large prostates.
However, this study had some limitations. Firstly, the study population of group C was too small (n=6). Although we performed a nonparametric comparison to avoid statistical errors, the power of the test might not have been enough owing to the small sample size. Secondly, all HoLEPs of group C were performed after overcoming the learning curve as aforementioned. Therefore, it is possible that the efficacy and safety parameters of group C were exaggerated.
Technical challenges are frequently encountered in HoLEP of extremely large prostates. Some researchers recommend that treatment of larger prostates be attempted only after overcoming the learning curve of HoLEP [4,21], and on the basis of our findings, we agree with this suggestion.
The most difficult point is the confusing orientation. Disorientation requires retraction of the scope for reorientation, which is highly time-consuming [3,16]. Moreover, a large prostate makes identification of the landmarks difficult [21,22]. Operators should always keep the threedimensional structure in mind, and preoperative TRUS may be helpful. Furthermore, the operator has to grasp the camera with the nondominant hand throughout the procedure to fix the view in a constant direction [16,21].
If the adenoma severely protrudes into the bladder lumen, the mucosal incision margin of the protruded prostatic adenoma is very close to the adjacent bladder wall. This increases the risk for potential injury to the bladder wall or sphincter. In those cases, measurement of the length from the penile meatus to the bladder neck with the scope and securing adequate length are necessary. Occasionally, the long prostatic urethral length in large prostates makes it difficult for the endoscope to reach the bladder neck. In that situation, some researchers recommend perineal urethrotomy to access the prostate safely [18,23]. Although we could perform surgery via a transurethral access in all patients in group C, these suggestions should be considered in inaccessible cases. Also, we have some experience with perineal access HoLEP in cases of extensive urethral stricture with bladder outlet obstruction.
Large prostates generally have an abundant blood supply, and bleeding together with clot formation can frequently restrict vision. Thus, meticulous hemostasis is required to ensure a clear view. To prevent capsule perforation owing to excessive lasing, focusing 2-3 mm from the bleeding point [22] or laser firing obliquely to the surface [24] may be helpful. Bleeding control is also important to ensure safe morcellation [25], and many researchers have recommended converting to adjuvant transurethral coagulation in uncontrolled bleeding situations.
Furthermore, identifying proper planes is difficult in large prostates, as satellite adenomas commonly exist. In these cases, efforts to identify the outermost capsular plane make enucleation more difficult and sometimes cause capsular perforation. Instead, it is safer to enucleate the main adenoma of the clear plane first and then to perform additional enucleation of the satellite adenomas. If residual adenoma tissues are found adjacent to the external sphincter, adjuvant TUR may be safer in terms of avoiding sphincter injury instead of hard enucleation. The extent of mucosal incision tends to be wider than that intended by the operator; hence, there is always a potential risk for sphincter injury.
Lastly, morcellation is also difficult owing to poor vision resulting from frequent bleeding events [5]. Therefore, meticulous bleeding control before morcellation is necessary, and the bladder should be expended sufficiently during the morcellation to prevent mucosal injury [26]. The aforementioned measurements of the bladder neck obtained with a cystoscope are also helpful to localize the blade at the proper depth; the morcellator should not be manipulated when the blade is not along the midline. To distinguish the adenoma from the bladder mucosa, the "swivel technique" can be helpful, as previously proposed [15]. The presence of hard nodules has been commonly reported [3,16,21,22]; herein, adjuvant TUR was performed for two cases (33.3%) owing to these nodules. Other authors have recommended additional surface incision with laser [21] or conversion to adjuvant TUR [22]. Close postoperative follow-up of the patients, with particular attention to urethral stricture, is recommended, because the total operation time is usually longer than that for small-tomoderate prostates.
Figures and Tables
Fig. 1
Technical challenges in enucleation of an extremely large prostate. (A) The enlarged adenomas frequently protrude into the urethral lumen; therefore, a bottleneck is commonly formed at the bladder neck level, and intravesical prostatic protrusion of the extremely enlarged prostate is also common. (B) Occasionally, insufficient cystoscopic length owing to the large volume of the prostate is encountered. The too narrow space and acute angle from the scope continuously disturb the cystoscopic view. (C) Owing to the presence of large and multiple adenoma nodules, finding a proper capsule plane is difficult, and the range of cystoscopic manipulation is always restricted. (D) Furthermore, pushing the enucleated adenoma tissue into the bladder may also be difficult. Scan this QR code to see the accompanying video, or visit www.kjurology.org or http://youtu.be/F77Hp8x4iZU.

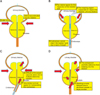
Table 1
Baseline characteristics

Values are presented as mean±standard deviation or number (%).
Group A, total prostate volume (TPV)<100 mL; group B, 100 mL≤TPV<200 mL; group C, TPV≥200 mL; IPSS, International Prostate Symptom Score; Qmax, maximal flow rate; PVR, postvoid residual; MCC, maximal cystometric capacity; PdetQmax, detrusor pressure at maximal flow rate.
a:Kruskal-Wallis or chi-square test. *p<0.05.
Table 2
Perioperative parameters and postoperative safety outcomes

Table 3
Comparison of the surgical outcome parameters

References
1. Elzayat EA, Habib EI, Elhilali MM. Holmium laser enucleation of the prostate: a size-independent new "gold standard". Urology. 2005; 66:5 Suppl. 108–113.
2. Kuntz RM, Lehrich K, Ahyai S. Does perioperative outcome of transurethral holmium laser enucleation of the prostate depend on prostate size? J Endourol. 2004; 18:183–188.
3. Tan AH, Gilling PJ. Holmium laser prostatectomy: current techniques. Urology. 2002; 60:152–156.
4. Shah HN, Mahajan AP, Sodha HS, Hegde S, Mohile PD, Bansal MB. Prospective evaluation of the learning curve for holmium laser enucleation of the prostate. J Urol. 2007; 177:1468–1474.
5. Seki N, Mochida O, Kinukawa N, Sagiyama K, Naito S. Holmium laser enucleation for prostatic adenoma: analysis of learning curve over the course of 70 consecutive cases. J Urol. 2003; 170:1847–1850.
6. Wilson LC, Gilling PJ, Williams A, Kennett KM, Frampton CM, Westenberg AM, et al. A randomised trial comparing holmium laser enucleation versus transurethral resection in the treatment of prostates larger than 40 grams: results at 2 years. Eur Urol. 2006; 50:569–573.
7. Montorsi F, Naspro R, Salonia A, Suardi N, Briganti A, Zanoni M, et al. Holmium laser enucleation versus transurethral resection of the prostate: results from a 2-center prospective randomized trial in patients with obstructive benign prostatic hyperplasia. J Urol. 2008; 179:5 Suppl. S87–S90.
8. Gupta N, Sivaramakrishna , Kumar R, Dogra PN, Seth A. Comparison of standard transurethral resection, transurethral vapour resection and holmium laser enucleation of the prostate for managing benign prostatic hyperplasia of >40 g. BJU Int. 2006; 97:85–89.
9. Tan A, Liao C, Mo Z, Cao Y. Meta-analysis of holmium laser enucleation versus transurethral resection of the prostate for symptomatic prostatic obstruction. Br J Surg. 2007; 94:1201–1208.
10. Kuntz RM, Lehrich K, Ahyai S. Transurethral holmium laser enucleation of the prostate compared with transvesical open prostatectomy: 18-month follow-up of a randomized trial. J Endourol. 2004; 18:189–191.
11. Reich O, Gratzke C, Stief CG. Techniques and long-term results of surgical procedures for BPH. Eur Urol. 2006; 49:970–978.
12. Kuntz RM, Lehrich K, Ahyai SA. Holmium laser enucleation of the prostate versus open prostatectomy for prostates greater than 100 grams: 5-year follow-up results of a randomised clinical trial. Eur Urol. 2008; 53:160–166.
13. Gilling PJ, Kennett KM, Fraundorfer MR. Holmium laser enucleation of the prostate for glands larger than 100 g: an endourologic alternative to open prostatectomy. J Endourol. 2000; 14:529–531.
14. Tan AH, Gilling PJ, Kennett KM, Frampton C, Westenberg AM, Fraundorfer MR. A randomized trial comparing holmium laser enucleation of the prostate with transurethral resection of the prostate for the treatment of bladder outlet obstruction secondary to benign prostatic hyperplasia in large glands (40 to 200 grams). J Urol. 2003; 170(4 Pt 1):1270–1274.
15. Kim M, Lee HE, Oh SJ. Technical aspects of holmium laser enucleation of the prostate for benign prostatic hyperplasia. Korean J Urol. 2013; 54:570–579.
16. Kuo RL, Paterson RF, Kim SC, Siqueira TM Jr, Elhilali MM, Lingeman JE. Holmium laser enucleation of the prostate (Ho-LEP): a technical update. World J Surg Oncol. 2003; 1:6.
17. Jeong CW, Oh JK, Cho MC, Bae JB, Oh SJ. Enucleation ratio efficacy might be a better predictor to assess learning curve of holmium laser enucleation of the prostate. Int Braz J Urol. 2012; 38:362–371.
18. Matlaga BR, Kim SC, Kuo RL, Watkins SL, Lingeman JE. Holmium laser enucleation of the prostate for prostates of 125 mL. BJU Int. 2006; 97:81–84.
19. Descazeaud A, Robert G, Azzousi AR, Ballereau C, Lukacs B, Haillot O, et al. Laser treatment of benign prostatic hyperplasia in patients on oral anticoagulant therapy: a review. BJU Int. 2009; 103:1162–1165.
20. Naspro R, Suardi N, Salonia A, Scattoni V, Guazzoni G, Colombo R, et al. Holmium laser enucleation of the prostate versus open prostatectomy for prostates >70 g: 24-month followup. Eur Urol. 2006; 50:563–568.
21. El-Hakim A, Elhilali MM. Holmium laser enucleation of the prostate can be taught: the first learning experience. BJU Int. 2002; 90:863–869.
22. Shah HN, Mahajan AP, Hegde SS, Bansal MB. Peri-operative complications of holmium laser enucleation of the prostate: experience in the first 280 patients, and a review of literature. BJU Int. 2007; 100:94–101.
23. Elzayat EA, Elhilali MM. Holmium laser enucleation of the prostate (HoLEP): the endourologic alternative to open prostatectomy. Eur Urol. 2006; 49:87–91.
24. Gomez Sancha F, Bachmann A, Choi BB, Tabatabaei S, Muir GH. Photoselective vaporization of the prostate (GreenLight PV): lessons learnt after 3500 procedures. Prostate Cancer Prostatic Dis. 2007; 10:316–322.
25. Fraundorfer MR, Gilling PJ. Holmium:YAG laser enucleation of the prostate combined with mechanical morcellation: preliminary results. Eur Urol. 1998; 33:69–72.
26. Hurle R, Vavassori I, Piccinelli A, Manzetti A, Valenti S, Vismara A. Holmium laser enucleation of the prostate combined with mechanical morcellation in 155 patients with benign prostatic hyperplasia. Urology. 2002; 60:449–453.
SUPPLEMENTARY MATERIALS
An accompanying video can be found in the 'urology in motion' section of the journal homepage (www.kjurology.org). The supplementary data can also be accessed by scanning a QR code located on the Fig. 1 of this article, or be available on YouTube (http://youtu.be/F77Hp8x4iZU).
Video clip 1
Insufficient cystoscopic length for reaching the bladder neck (http://youtu.be/F77Hp8x4iZU)
Video clip 2
Difficulty in identifying the ureteral orifice owing to the restricted visual angle (http://youtu.be/-KktjeVgoAI).
Video clip 3
Risk of bladder mucosal injury owing to the adjacent bladder wall (http://youtu.be/GULbh2btZRo).
Video clip 4
Multiple capsular planes caused by the multiple adenomas (http://youtu.be/_SZcxk6OMCo).
Video clip 6
A hard nodule requiring adjuvant transurethral resection (http://youtu.be/Hp2863-nM4c).




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download