Abstract
To describe the novel technique of ventral inlay substitution urethroplasty for the management of male anterior urethral stricture disease. A 58-year-old gentleman with multifocal bulbar stricture disease measuring 7 cm in length was treated using a ventral inlay substitution urethroplasty. A dorsal urethrotomy was created, and the ventral urethral plated was incised. The edges of the urethral plate were mobilized without violation of the ventral corpus spongiosum. A buccal mucosa graft was harvested and affixed as a ventral inlay to augment the caliber of the urethra. The dorsal urethrotomy was closed over a foley catheter. No intraoperative or postoperative complications occurred. Postoperative imaging demonstrated a widely patent urethra. After three years of follow-up, the patient continues to do well with no voiding complaints and low postvoid residuals. Ventral inlay substitution urethroplasty appears to be a safe and feasible technique for the management of bulbar urethral strictures.
A 58-year-old African American gentleman presented with a history of idiopathic bulbar urethral stricture disease. He had been managed with multiple transurethral dilation and incision procedures for over a decade. He reported persistent urinary frequency and a weakened force of stream. Urodynamics testing had been performed previously and demonstrated a high-pressure, low-flow pattern with normal bladder capacity. He noted chronic erectile dysfunction with intermittent success using phosphodiesterase-5 (PDE5) inhibitors. His additional medical history included sarcoidosis, anemia of chronic disease, gout, and hypertension. The physical exam demonstrated normal vital signs and normal external male genitalia. Urinalysis, prostate-specific antigen levels, and testosterone levels were within normal limits. Baseline hemoglobin was 10.9 g/dL and creatinine was 1.5 mg/dL. Postvoid residual urine volume (PVR) was 104 mL. Retrograde urethrogram and cystoscopic examination demonstrated a multifocal proximal bulbar stricture (Fig. 1).
After extensive counseling, the patient was taken to the operating room for definitive management of his stricture with substitution urethroplasty. He was placed into an unexaggerated lithotomy position. A perineal approach was utilized. The bulbar urethra was circumferentially mobilized without sacrifice of the bulbar arteries (Fig. 2A). The area of stricture was confirmed by intraoperative flexible cystoscopy. Four 4-0 Vicryl stay stitches were placed in the urethra to allow for 180 degrees of rotation, providing excellent access to the dorsal urethra. The dorsal aspect of the urethra was opened sharply at the 12 o'clock position between the stay sutures (Fig. 2B). The length of the urethrotomy was extended until the distal and proximal urethral segments easily accommodated 26-Fr and 30-Fr calibration, respectively (Fig. 2C). Flexible cystoscopy then confirmed a normal appearance of the prostate and urinary bladder. The stricture ultimately measured 7 cm in length, and the urethral plate measured 1.2 cm in width. A buccal mucosal graft measuring 2.2 cm in width was harvested from the inner left cheek in standard fashion. The graft was then defatted and fenestrated.
Through the dorsal urethrotomy, the ventral urethral plate was sharply incised in the midline without significant violation of the spongiosum. The wings of the opened plate were mobilized with sharp dissection to allow for an elliptical defect. The graft was sewn into place by using two separate 5-0 PDS sutures in running fashion (Fig. 2D). A lubricated 16-Fr silicone Foley catheter was then placed under vision across the repaired area (Fig. 2E). The dorsal urethra was reapproximated with running 5-0 PDS sutures (Fig. 2F). The perineum was closed in three layers of absorbable sutures. Overall blood loss was minimal and the patient suffered no intraoperative complications.
The patient's postoperative imaging 3 weeks after the procedure demonstrated a patent, wide-caliber urethra (Fig. 3). After 3 years of follow-up, he was voiding with a strong stream and with minimal PVR (<10 mL) at every visit. He expressed no complaints concerning his voiding. He has not required any further interventions to address his stricture. He continued to use PDE5 inhibitors for his erectile dysfunction with good results.
Bulbar urethral strictures are a common reason for presentation to a reconstructive urologist [1]. Historically, numerous surgical approaches have been used to treat these lesions [2,3]. Depending on the length and location of the stricture as well as the experience and preference of the surgeon, strictures in the bulbar urethra may be approached in an excisional manner or with tissue substitution. Although excision with primary anastomosis can be applied to extensive proximal strictures, this is controversial and challenging for most practicing urologists [4]. For longer or more complicated strictures, substitution techniques utilizing grafts or flaps are often employed. In general, substituted tissue is transferred into the urethra as either an onlay or inlay. In onlay procedures, the urethra and its overlying spongiosium are generally opened throughout the length of the stricture. The graft material is then either substituted in place of both the mucosa and sponge together (dorsal onlay) or in place of the mucosa only with the transected sponge being closed over the repair (ventral onlay). In contrast, in inlay procedures, only the mucosa of interest is opened throughout the length of the stricture, with the corresponding sponge remaining largely undisturbed. The mucosal edges are then usually undermined to some degree, and the graft material is then sewn to the edges of the mucosa.
In general, bulbar strictures in excess of 2 cm are most commonly treated with ventral onlay of buccal mucosa. Although this is a fairly straightforward procedure anatomically, it does require disruption of the ventral spongiosum. This disruption is avoided by using the ventral inlay technique described in this article. It is important to recognize that this vascular tissue bed becomes much more concentrated ventrally as one moves from the distal to the proximal urethra [5]. Division of this network of vessels can contribute to intraoperative blood loss that may limit visualization. Additionally, there is a theoretical benefit in terms of graft support if more of this vascular tissue could be spared. Furthermore, suture reapproximation of the spongiosum after a ventral onlay could create a furled lie of the graft, although this has not been established to the author's knowledge. For these reasons, the ventral inlay technique seems to have several potential advantages.
Recently, Andrich and Mundy [6] described a nontransecting anastomotic bulbar urethroplasty. This work builds on the concept of a "stricturoplasty" that takes advantage of the Heineke-Mikulicz principle. This was previously described by James Pierce in 1962 and was resurrected by Lumen et al. [7] in 2010. Andrich and Mundy [6] wisely point out that some patients may already have impaired retrograde blood flow (e.g., prior hypospadias repair), and preservation of vascularity seems prudent. The ventral inlay technique that we have described here is designed to minimize disruption of the spongiosum while providing a well-vascularized bed for the graft. Whereas this may not be well suited to every patient with stricture disease (i.e., patients with full thickness spongiofibrosis), for well-selected patients, it seems to offer several theoretical benefits.
Additionally, patients presenting for definitive repair of urethral stricture may require further urethral procedures for other reasons later in life. For example, many patients will go on to be screened and possibly treated for prostate cancer. Despite improvements in delivery of radiation and technical performance of radical prostatectomy, a subset of these men may require placement of an artificial urinary sphincter for treatment of incontinence [8]. It is logical to assume that maximizing the potential of the urethra to resist compression-induced erosion would, therefore, be beneficial. This concept has been reinforced by utilizing surgical maneuvers to preserve the bulbar arteries at the time of anastomotic urethroplasty. Additionally, Andrich and Mundy [6] point out that minimizing spongiosal trauma may also be of benefit to patients who require a subsequent urethroplasty. Thus, in the era of minimally invasive surgery, it seems prudent to preserve as much blood flow as possible as long as this does not compromise the treatment of the patient.
Since this is the first description of an approach to bulbar stricture disease and a solitary case, it does not serve to establish superiority over ventral onlay substitution. However, our report demonstrates the feasibility of substitution urethroplasty for bulbar strictures with minimal disruption to the ventral spongiosum. One may argue that ventral onlay is often done in the proximal bulb, because this technique obviates the disruption associated with circumferential urethral mobilization. Alternatively, dorsal onlay could be performed as suggested by Andrich and Mundy [6]. However, a few key points deserve consideration. Barbagli et al. [9] reviewed ventral, dorsal, and lateral graft onlays for bulbar strictures and showed no difference in overall success. More recently, Figler et al. [10] also reviewed the impact of graft position and once again showed no appreciable differences between dorsal and ventral placement. However, what if the ventral onlay technique only failed to show superiority owing to division of the spongiosum? Barbagli's group suggested that "ventral placement of the graft is more efficacious in the proximal part of the bulbar urethra, where the spongiosum tissue is thicker and better vascularized." Additionally, they felt that graft failure may stem from poor inosculation from insufficient vascularity of the graft bed, which would seem more likely when the graft is placed on the thinner dorsal spongiosum or on a divided ventral spongiosum. The approach described here takes advantage of having a graft in direct contact with an undisturbed bed of spongiosum at its thickest location.
To the authors' knowledge, this represents the first description of a ventral inlay in the treatment of urethral stricture disease. Although further study will be required to determine the role of this type of repair in the reconstructive urologist's armamentarium, feasibility has been established and a logical rationale exists for considering this type of approach.
Figures and Tables
Fig. 1
Preoperative workup. (A) Retrograde urethrogram demonstrating multifocal stricturing of the proximal bulbar urethra. (B) Cystoscopy showing narrowing and scarring of a long segment of the bulbar urethra.
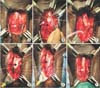
Fig. 2
Key surgical images from ventral inlay substitution urethroplasty technique. (A) Complete exposure of the bulbar urethra with the urethra in its normal anatomic orientation (white arrow, ventral urethra). (B) Mobilized urethra rotated 180 degrees and with stay sutures in place, dorsal urethrostomy created at 12 o'clock (black arrow, dorsal urethra; white arrow, Vicryl stay stitch). (C) Dorsal urethrostomy completed along the entire length of the stricture. (D) Ventral urethral plate incised and urethral plate wings mobilized; buccal mucosal graft (BMG) sewn into place (black arrow, BMG inlay within ventral urethra). (E) Catheter placed across the area of repair (black arrow, Foley catheter). (F) Urethra closed dorsally and rotated back into anatomic position after repair (white arrow, ventral urethra).
References
1. Santucci RA, Joyce GF, Wise M. Male urethral stricture disease. J Urol. 2007; 177:1667–1674.
2. DeWeerd JH. Heineke-Mikulicz principle applied to retropubic revision of the vesical neck. J Urol. 1966; 95:368–373.
3. Lumen N, Monstrey S, Goessaert AS, Oosterlinck W, Hoebeke P. Urethroplasty for strictures after phallic reconstruction: a single-institution experience. Eur Urol. 2011; 60:150–158.
4. Terlecki RP, Steele MC, Valadez C, Morey AF. Grafts are unnecessary for proximal bulbar reconstruction. J Urol. 2010; 184:2395–2399.
5. Carroll PR, Dixon CM. Surgical anatomy of the male and female urethra. Urol Clin North Am. 1992; 19:339–346.
6. Andrich DE, Mundy AR. Non-transecting anastomotic bulbar urethroplasty: a preliminary report. BJU Int. 2012; 109:1090–1094.
7. Lumen N, Hoebeke P, Oosterlinck W. Ventral longitudinal stricturotomy and transversal closure: the Heineke-Mikulicz principle in urethroplasty. Urology. 2010; 76:1478–1482.
8. Kim PH, Pinheiro LC, Atoria CL, Eastham JA, Sandhu JS, Elkin EB. Trends in the use of incontinence procedures after radical prostatectomy: a population based analysis. J Urol. 2013; 189:602–608.
9. Barbagli G, Palminteri E, Guazzoni G, Montorsi F, Turini D, Lazzeri M. Bulbar urethroplasty using buccal mucosa grafts placed on the ventral, dorsal or lateral surface of the urethra: are results affected by the surgical technique? J Urol. 2005; 174:955–957.
10. Figler BD, Malaeb BS, Dy GW, Voelzke BB, Wessells H. Impact of graft position on failure of single-stage bulbar urethroplasties with buccal mucosa graft. Urology. 2013; 82:1166–1170.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download