Abstract
Purpose
To evaluate changes in differential renal function (DRF), as a functional outcome, in children who underwent redo pyeloplasty for management of failed pyeloplasty and to examine the factors that affect functional outcomes.
Materials and Methods
Between January 2002 and November 2010, a total of 18 patients who underwent redo pyeloplasty for persistent ureteropelvic junction obstruction after failed pyeloplasty were enrolled in this study. We assessed perioperative factors and evaluated changes in renal cortical thickness (RCT), renal function, and hydronephrosis by use of serial ultrasound and diuretic renography.
Results
The mean follow-up period was 44.83±28.86 months. After redo pyeloplasty, prevention of further functional deterioration was observed in only 12 of the 18 patients. After dividing the patients according to this observation, we discovered significant differences in both change in DRF (dDRF) and change in RCT (dRCT) (difference between before and after initial pyeloplasty) between the two groups (p<0.001). Additionally, we noted a significant positive correlation between dRCT and dDRF. All patients showed improvements in hydronephrosis grade and relief of symptoms compared with before redo pyeloplasty.
Conclusions
Redo pyeloplasty should be considered in cases of failed pyeloplasty to preserve renal function and obtain relief from symptoms. If patients show severe deterioration of DRF or a decrease in RCT after initial pyeloplasty, preservation of DRF in these patients after redo pyeloplasty could be difficult. Therefore, redo pyeloplasty should be performed before severe deterioration of DRF or decrease in RCT.
If left untreated, ureteropelvic junction obstruction (UPJO) can lead to hydronephrosis and progressive impairment of renal function. With success rates exceeding 98%, Anderson-Hynes dismembered pyeloplasty has become the gold standard for treating primary UPJO [1]. Although pyeloplasty failure is uncommon, it does occur, requiring secondary surgical intervention [2,3]. Currently, several studies have reported on the high success rate of redo pyeloplasty [4,5]. However, to our knowledge, the factors affecting functional outcomes after redo pyeloplasty have not yet been reported. Accordingly, the aim of this retrospective study was to evaluate changes in differential renal function (DRF), as a functional outcome, in children who underwent redo pyeloplasty for the management of failed pyeloplasty and to outline the factors associated therewith. Our observations may help to improve functional outcomes of redo pyeloplasty.
With approval from the Institutional Review Broad of Severance Hospital (4-2014-0081), medical records were obtained from a database of patients who had undergone redo pyeloplasty between January 2002 and November 2010 at Severance Hospital in Seoul, Korea. During this period, a total of 21 children underwent redo pyeloplasty by a single surgeon (S.W.H.) at Sevrance Hospital. The initial pyeloplasties were performed at our institution in 11 children, and the remaining procedures were performed at other institutions.
Information on preoperative DRF and renal cortical thickness (RCT) was not available for 3 patients who had undergone renal scintigraphy or ultrasound at other institutions, and these patients were excluded from the analysis. Finally, a total of 18 patients were enrolled in this study.
Failure of initial pyeloplasty was judged by either obstructive symptoms or signs. The decision to perform redo pyeloplasty depended on the presence of symptoms (e.g., urinary tract infection, flank pain), functional loss (deterioration of DRF of more than 5%), and an aggravated obstruction pattern on a renogram or a huge urinoma.
The patients were followed up postoperatively by use of serial ultrasound and renal scintigraphy for evaluating long-term functional outcomes. Follow-up ultrasound was performed at 4 to 6 weeks after the operation and was then repeated every 1 to 6 months thereafter, according to the results of a previous study. The degree of hydronephrosis was graded from 0 to 4 according to the Society for Fetal Urology (SFU) classification scheme [6]. Renal scintigraphy was performed 6 months after the operation and was repeated if needed.
DRF was assessed by using either 99mTc-dimercaptosuccinic acid or 99mTc-mercaptoacetyltriglycine. RCT was measured in the sagittal plane at the level of the midkidney, as described by Moghazi et al. [7]. The measurement was obtained over the medullary pyramid, perpendicular to the capsule, and as the shortest distance from the base of the medullary pyramid to the renal capsule.
Statistical comparisons of continuous variables in patient demographics were carried out by using the Mann-Whitney U-test. Categorical variables were calculated by using Fisher exact test. The Wilcoxon signed rank test was used for paired comparisons of before and after the operation. PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. All p-values less than 0.05 were considered statistically significant.
The characteristics of the patients enrolled in this study af ter initial pyeloplasty are summarized in Table 1. All patients showed at least persistent or mild increases of hydronephrosis on ultrasound, with results on postoperative renal scintigraphy consistent with an obstruction. The mean interval between operations (between initial pyeloplasty and redo pyeloplasty) was 13.67±10.33 months. The causes of redo pyeloplasty included persistent obstruction on renography related to worsening hydronephrosis or a huge urinoma on ultrasound or the development of symptomatic obstruction, such as urinary tract infection and recurrent pain. With a mean follow-up period of 44.83±28.86 months, unilateral obstruction was resolved in 18 patients after redo pyeloplasty.
Redo pyeloplasty was performed in 2 patients owing to a huge urinoma. Both showed increased drain output and a huge urinoma on an ultrasound after the first pyeloplasty. Thus, we first attempted ureteral stent insertion, which failed. Within about 1 week, redo pyeloplasty was performed.
A stented redo pyeloplasty was performed in 11 patients. Whether to use a stent was determined on the basis of viability and fibrotic changes in the UPJ, as well as the presence of perinephric tissue. Stents were removed after a mean of 29.6±19.1 days. Dismembered-type pyeloplasty was performed in 13 patients.
DRF on renal scintigraphy worsened after the initial pyeloplasty in 6 patients, who showed deterioration of renal function (decrease of more than 5%); was stable in 11 patients; and slightly increased in 1 patient. The mean DRF of diseased kidneys before and after initial pyeloplasty was 45.77%±6.05% and 38.72%±15.44%, respectively. At approximately 6 months after redo pyeloplasty, the mean DRF increased to only 40.50%±15.12%, a difference that was not significant. After redo pyeloplasty, prevention of further functional deterioration was recorded in two-thirds of the patients but not in the remaining one-third (Fig. 1).
Before redo pyeloplasty, 14 patients were hydronephrosis grade 4 and the others were hydronephrosis grade 3. When we evaluated hydronephrosis grade with serial ultrasound after redo pyeloplasty, all patients showed an improvement in hydronephrosis grade compared with that before redo pyeloplasty.
We divided all 18 patients into two groups according to change in DRF. Six patients showed a decrease of more than 5% DRF compared with initial DRF. The mean ages were 55.50±72.1 months in the decrease in DRF group and 55.50±47.15 months in the no decrease in DRF group (p=0.616). The mean follow-up duration between operations was 13.66±12.40 months in the decrease in DRF group and 13.66±6.77 months in the no decrease in DRF group (p=0.682). The mean DRF before initial pyeloplasty was 45.16%±5.60% in the decrease in DRF group and was not significantly different from that (46.08%±6.41%) in the no decrease in DRF group (p=0.604). Gender, hydronephrosis grade, and operation type (dismembered vs. nondismembered; stented vs. unstented) were not statistically different between the two groups.
dDRF was calculated as the difference in DRF between before and after initial pyeloplasty. In the decrease in DRF group, the mean dDRF was -23.00%±12.31%. In the no decrease in DRF group, the mean dDRF was 0.91%±4.62%. In the decrease in DRF group, DRF was significantly decreased between before and after initial pyeloplasty (p=0.028); in the no decrease in DRF group, the difference was not significant (p=0.397). Overall, dDRF differed significantly between the two groups (p<0.001).
dRCT was calculated as the difference in RCT between before and after initial pyeloplasty. In the decrease in DRF group, the mean dRCT (-3.56±2.9 mm) was higher than that in the no decrease in DRF group (-0.41±0.27 mm), a significant difference between the two groups (p<0.001).
Additionally, we calculated rDRF as the difference in DRF between before and after redo pyeloplasty, as a reflection of the level of recovery of DRF after redo pyeloplasty. In the decrease in DRF group, the mean rDRF was 1.16%±2.99%. In the no decrease in DRF group, it was 2.08%±3.23%. The difference in rDRF between the two groups was not significant (p=0.541) (Table 2).
Finally, we noted a significant positive correlation between dRCT and dDRF (differences between before and after the initial operation; p<0.001; R2 linear=0.716). Patients without deterioration of renal function showed almost no change in RCT. Meanwhile, patients with a decline in DRF of more than 5% showed greater decreases in RCT (Fig. 2).
During the follow-up period, we observed one complication associated with redo pyeloplasty. The patient showed no change in hydronephrosis grade (SFU grade 3) and reported experiencing flank pain after redo pyeloplasty. Therefore, we performed double J stent insertion at 1 month after the redo operation. We removed the stent after 1 month and after all symptoms had disappeared.
Since Anderson and Hynes reported on the first successful dismembered pyeloplasty in 1891, many advances have been made in the surgical management of UPJO. However, the basic surgical principles have remained largely the same, including the meticulous preservation of the ureteral blood supply, construction of a widely patent and watertight anastomosis, and careful tissue handling. These principles have allowed dismembered pyeloplasty to be successful in relieving UPJO in up to 98% of cases [1].
Even when patients are optimally managed, however, pyeloplasty fails in a small but steady proportion of patients. Several treatment approaches exist for secondary UPJO af ter failed pyeloplasty. Among them, redo pyeloplasty, by use of both open and minimally invasive techniques, appears to be the most effective, with success rates higher than 90% among pediatric patients [8,9,10,11]. However, no reports yet exist concerning changes in renal function after redo pyeloplasty. Also, factors that can inform the functional outcome of redo pyeloplasty remain undetermined. Therefore, in the present study, we set out to evaluate changes in DRF and RCT by use of serial renal scintigraphy and ultrasound. In doing so, we found that, after redo pyeloplasty, DRF on renal scintigraphy was similar to that after failed pyeloplasty, reflecting the difficulties of recovering initial renal function.
In previous studies, researchers noted that DRF significantly improved in children with immediate or delayed pyeloplasty [12,13]. On the other hand, another study reported that Anderson-Hynes pyeloplasty had no effect on renal function after surgery and that the increase in renal function at follow-up in infants might be attributable to their normal growth potential [14]. In light of our results, patients should not expect dramatic improvements in renal function after redo pyeloplasty.
Nonetheless, prior to conducting this study, we assumed that recovery of renal function after redo pyeloplasty would reflect preservation or improvement in initial DRF. However, when we compared two groups of patients divided according to a decrease in DRF of more than 5% of initial DRF, dDRF was shown to be a factor that significantly contributed to functional recovery after redo pyeloplasty in pediatric patients. We discerned this to mean that severe reductions in renal function after an initial surgery may greatly affect the likelihood of recovering initial renal function after redo pyeloplasty.
For detecting severely reduced DRF after a failed pyeloplasty, we attempted to assess RCT as another factor of recoverability of renal function. Herein, dRCT was shown to be a significant factor that affected the functional outcome of redo pyeloplasty. Previously, Harraz et al. [15] reported that, after the relief of obstruction, there is a tendency for renal function to recover, irrespective of nephron mass, as determined by cortical thickness. They speculated that healthy nephrons might explain the ability of the kidneys to recover. Other investigators have also reported RCT as a powerful predictor of renal function [16,17]. In connection with these reports, we found a positive correlation between dDRF and dRCT in patients who experienced a failed pyeloplasty. Accordingly, we think that dRCT could be a predictor of dDRF in patients scheduled to undergo redo pyeloplasty, which may help physicians in predicting the likelihood of recovering initial renal function thereafter. Although RCT on ultrasound could present bias in the outcome measure, standardized measurement of RCT with serial ultrasound would be helpful to determine the benefits of a secondary operation.
Additionally, we evaluated changes in hydronephrosis after redo pyeloplasty. Most patients showed an improvement in hydronephrosis after redo pyeloplasty, although normalization was rare. In primary pyeloplasty, Park et al. [18] reported that both symptomatic cases and delayed improvements in hydronephrosis (i.e., up to 6 months) were identified as risk factors for lack of normalization. These factors may reflect a reduced resilience of the pelvis. Therefore, the possibility of normalization after redo pyeloplasty seems to be lower than that after initial pyeloplasty.
Nevertheless, we must acknowledge the limitations of our report, including the inherent drawbacks of the retrospective design and the lack of bias control. Also, the accuracy of using renal scintigraphy as a measure of renal function is in question [19,20]. Although large-scale studies are needed to draw more definitive conclusions, these are difficult owing to the very low failure rate of pyeloplasty. As strengths of our study, however, the data in our series were collected from patients who underwent redo surgeries performed by a single surgeon and included many variables that might predict DRF recoverability after redo pyeloplasty. Accordingly, we believe that our study is important to establishing the concept of a renal functional outcome for predicting improvement after redo pyeloplasty. Such a concept would better equip physicians for proper counseling of patients before surgery and for making successful surgical decisions.
Redo pyeloplasty should be considered in cases of failed pyeloplasty in order to preserve renal function and to offer relief from symptoms. In patients who underwent redo pyeloplasty, dDRF and dRCT were shown to be factors affecting the functional outcomes of this procedure. Meanwhile, in patients who show severe deteriorations in DRF or decreases in RCT after initial pyeloplasty, recovery of initial DRF after redo pyeloplasty may be difficult. Therefore, redo pyeloplasty should be performed before severe deterioration of DRF or decreases in RCT.
Figures and Tables
Fig. 1
Changes in differential renal function (DRF) after initial and redo pyeloplasty on renal scintigraphy. (A) Patients that final DRF were not decreased more than 5% of the initial DRF. (B) Patients that final DRF were decreased more than 5% of the initial DRF.

Fig. 2
Correlation between change in renal cortical thickness (dRCT) and change in differential renal function (dDRF) from before to after initial pyeloplasty.

Table 1
Characteristics of patients who underwent redo pyeloplasty

Table 2
Comparison of patients grouped according to decrease in differential renal function (DRF) of more than 5% (initial vs. final)
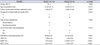
Values are presented as mean±standard deviation.
Group 1, patients in whom final DRF did not decrease by more than 5% of the initial DRF; group 2, patients in whom final DRF decreased by more than 5% of the initial DRF; RCT, renal cortical thickness.
A p-value was analyzed by Mann Whitney U-test , Fisher exact test.
a: Difference in DRF between before and after redo pyeloplasty. b: Difference in DRF between before and after initial pyeloplasty. c: Difference in RCT between before and after initial pyeloplasty. *: p<0.05 by Wilcoxon signed rank test.
References
1. Salem YH, Majd M, Rushton HG, Belman AB. Outcome analysis of pediatric pyeloplasty as a function of patient age, presentation and differential renal function. J Urol. 1995; 154:1889–1893.
2. Lim DJ, Walker RD 3rd. Management of the failed pyeloplasty. J Urol. 1996; 156(2 Pt 2):738–740.
3. Thomas JC, DeMarco RT, Donohoe JM, Adams MC, Pope JC 4th, Brock JW 3rd. Management of the failed pyeloplasty: a contemporary review. J Urol. 2005; 174:2363–2366.
4. Anderson JC, Hynes W. Retrocaval ureter; a case diagnosed pre-operatively and treated successfully by a plastic operation. Br J Urol. 1949; 21:209–214.
5. Ng CS, Yost AJ, Streem SB. Management of failed primary intervention for ureteropelvic junction obstruction: 12-year, single-center experience. Urology. 2003; 61:291–296.
6. Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Pediatr Radiol. 1993; 23:478–480.
7. Moghazi S, Jones E, Schroepple J, Arya K, McClellan W, Hennigar RA, et al. Correlation of renal histopathology with sonographic findings. Kidney Int. 2005; 67:1515–1520.
8. Braga LH, Lorenzo AJ, Skeldon S, Dave S, Bagli DJ, Khoury AE, et al. Failed pyeloplasty in children: comparative analysis of retrograde endopyelotomy versus redo pyeloplasty. J Urol. 2007; 178:2571–2575.
9. Helmy TE, Sarhan OM, Hafez AT, Elsherbiny MT, Dawaba ME, Ghali AM. Surgical management of failed pyeloplasty in children: single-center experience. J Pediatr Urol. 2009; 5:87–89.
10. Seixas-Mikelus SA, Jenkins LC, Williot P, Greenfield SP. Pediatric pyeloplasty: comparison of literature meta-analysis of laparoscopic and open techniques with open surgery at a single institution. J Urol. 2009; 182:2428–2432.
11. Romao RL, Koyle MA, Pippi Salle JL, Alotay A, Figueroa VH, Lorenzo AJ, et al. Failed pyeloplasty in children: revisiting the unknown. Urology. 2013; 82:1145–1147.
12. Heinlen JE, Manatt CS, Bright BC, Kropp BP, Campbell JB, Frimberger D. Operative versus nonoperative management of ureteropelvic junction obstruction in children. Urology. 2009; 73:521–525.
13. Szavay PO, Luithle T, Seitz G, Warmann SW, Haber P, Fuchs J. Functional outcome after laparoscopic dismembered pyeloplasty in children. J Pediatr Urol. 2010; 6:359–363.
14. Materny J, Mazurkiewicz I, Gawrych E, Birkenfeld B, Zorga P. Does Hynes-Anderson pyeloplasty improve renal function? Ann Acad Med Stetin. 2010; 56:95–102.
15. Harraz AM, Helmy T, Taha DE, Shalaby I, Sarhan O, Dawaba M, et al. Changes in differential renal function after pyeloplasty in children. J Urol. 2013; 190:4 Suppl. 1468–1473.
16. Beland MD, Walle NL, Machan JT, Cronan JJ. Renal cortical thickness measured at ultrasound: is it better than renal length as an indicator of renal function in chronic kidney disease? AJR Am J Roentgenol. 2010; 195:W146–W149.
17. Kaplon DM, Lasser MS, Sigman M, Haleblian GE, Pareek G. Renal parenchyma thickness: a rapid estimation of renal function on computed tomography. Int Braz J Urol. 2009; 35:3–8.
18. Park K, Baek M, Cho SY, Choi H. Time course of hydronephrotic changes following unilateral pyeloplasty. J Pediatr Urol. 2013; 9(6 Pt A):779–783.
19. Bhat GS, Maregowda S, Jayaram S, Siddappa S. Is renal biopsy a better predictor of the outcome of pyeloplasty in adult ureteropelvic junction obstruction? Urology. 2012; 79:321–325.
20. Rosen S, Peters CA, Chevalier RL, Huang WY. The kidney in congenital ureteropelvic junction obstruction: a spectrum from normal to nephrectomy. J Urol. 2008; 179:1257–1263.
EDITORIAL COMMENT
Reoperation is a psychologically large burden on the operator, and the reoperation itself is more difficult than the initial operation because of the adhesive surgical field and poor tissue condition of the renal pelvis and ureter. Complicated surgical techniques such as flap surgery or ureterocalicostomy should be applied in some cases. Owing to the rarity of reoperation cases, knowledge about redo pyeloplasty has usually been gained through the experience of an individual surgeon. Thus, this study is valuable and very informative for showing detailed clinical data [1]. I would like to make some comments in the aspect of clinical practice.
First, after initial pyeloplasty, the postoperative result is not as simple and conclusive as "surgical failure." Sometimes, it is not easy for the surgeon to decide on reoperation. Discrepancies may exist in the imaging studies between the sonographic findings and excretion and renal function in the diuretic renogram. Some patients show delayed excretion despite moderate improvement of hydronephrosis. In particular, postoperative transient hydronephrosis could be present and a "wait-and-see" approach is considered because most cases improve spontaneously. In case No. 1, differential renal function (DRF) was 46% and hydronephrosis was grade 3; thus, the "wait-and-see" approach could be considered if the patient did not show flank pain or urinary tract infection.
Second, in a very poorly functioning kidney, maybe as the result of obstruction or infection, is it meaningful to perform redo pyeloplasty? We should not expect functional improvement by the relief of obstruction. In cases No. 10 and 15, the DRF was only 1% and 6%, respectively; thus, functional recovery was not expected in these cases [2]. In my experience, functional improvement can be achieved only in the case of an acute high-grade obstruction. Double J stenting and follow-up evaluation of functional change could be an option to predict functional restoration after the operation.
Finally, the author concluded that recovery of DRF after redo pyeloplasty is difficult in patients who show severe deteriorations in DRF or a decrease in renal cortical thickness after the initial pyeloplasty. The title of this article implicates the author's conclusive mind that delayed redo pyeloplasty fails to recover lost renal function. The authors suggest that one should not hesitate to perform reoperation in cases of postoperative findings such as sonographic changes and loss of renal function. Although it is not easy for surgeons to recommend reoperation during follow-up, it is worse to delay the decision for reoperation in cases showing definite deterioration. Eventually, the deterioration causes superimposed urinary tract infection and flank pain and finally leads to decreased renal function.
We acknowledge the limitations of this study, especially the unaccounted for compounding surgical factors such complicated surgical field conditions and the location and degree of stricture, which were unavoidable owing to the study design. Despite this limitation, we believe that these data provide us deep insight into redo pyeloplasty.
References
1. Chung DY, Hong CH, Im YJ, Lee YS, Kim SW, Han SW. Delayed redo pyeloplasty fails to recover lost renal function after failed pyeloplasty: early sonographic changes that correlate with a loss of differential renal function. Korean J Urol. 2015; Forthcoming.
2. Park S, Ji YH, Park KH, Han DH, Kim KS. Difference in results of ultrasonography and diuretic renograms after pyeloplasty in children with ureteropelvic junction obstruction. Korean J Urol. 2009; 50:596–601.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download