Abstract
Purpose
To evaluate the perioperative, functional, and oncological outcomes of renal cryoablation (RC) of small renal masses (SRMs) performed in Korea University Hospital.
Materials and Methods
We reviewed an Institutional Review Board-approved database of 70 patients who underwent RC and were followed up for a minimum of 3 months by a single surgeon in Korea University Hospital from August 2007 to May 2014. Among these patients, 68 patients (79 renal masses) were enrolled in our research. We evaluated perioperative, functional, and oncologic outcomes of RC.
Results
A total of 68 patients (79 renal masses) underwent RC in our institution. The mean age of the patients was 62.0 years. The mean tumor size was 2.25 cm. Among the 59 patients who underwent laparoscopic surgery, only 1 patient (1.47%) was converted to open surgery. No other perioperative complications occurred. The mean preoperative and 1-month postoperative estimated glomerular filtration ratio (eGFR) were 71.8 and 68.3 mL/min/1.73 m2, respectively (p=0.19). The mean 1-year postoperative eGFR was 65.0 mL/min/1.73 m2 (p=0.25). The mean follow-up period was 59.76 months (range, 3-119 months). Local tumor recurrence occurred in eight tumors (15.4%; a total of 52 renal cell carcinomas). Concerning treatment in the patients with recurrence, five patients underwent re-treatment and three patients are under active surveillance. None of the eight patients who experienced local recurrence had additional recurrence or tumor progression during the follow-up period. In our study, the recurrence-free rate was 83.0% and the cancer-specific survival rate was 100%. Moreover, the 5- and 10-year overall survival rates were both 100%.
According to the National Cancer Center in Korea, a total of 3,989 patients newly diagnosed with renal cell carcinoma (RCC) accounted for 1.8% of all cancer in 2011. This is an increase of about 6.2% per year, an increase that is higher than in the United States and Europe [1]. Because of the widespread use of abdominal imaging, the detection of asymptomatic small renal masses (SRMs) is increasingly prevalent [2]. For the management of these masses, partial nephrectomy (PN) is considered the gold standard of care [3]. However, PN is associated with perioperative complications in about 20% of cases [4,5], which cause significant morbidity [5]. In addition, serial studies of laparoscopic partial nephrectomy (LPN) report higher perioperative and postoperative complication rates than with open PN, which result in more ischemic time and longer hospital stays [6,7,8].
With the increasing application of minimally invasive surgery, several energy-based tissue ablation technologies including cryoablation are being investigated. RC is a recommended treatment option in specific populations, including patients who are elderly or have multiple comorbidities, solitary kidneys, ipsilateral multiple renal tumors, or bilateral renal tumors and patients who do not choose active surveillance [9,10,11].
Currently, long-term follow-up studies of RC are rare, especially in Korea. Thus, we report our 10-year experience with RC of SRMs in a single university hospital, which includes the evaluation of perioperative, functional, and oncological outcomes.
We reviewed an Institutional Review Board-approved database of 70 patients who underwent RC and were followed up for a minimum of 3 months by a single surgeon in Korea University Hospital from August 2007 to May 2014. Among these patients, 68 patients (79 renal masses) were enrolled in our study. Two patients were excluded from study. Of the two excluded patients, one patient was diagnosed with angiomyolipoma in the preoperative period and underwent laparoscopic renal cryoablation (LRC) and the other patient was lost to follow-up. Absolute indications for RC in our institution included renal tumors in a solitary kidney or chronic kidney disease (CKD), bilateral renal tumors, ipsilateral multiple renal tumors, and hereditary renal tumors. Elective indications included age (older than 70 years), multiple comorbidities (American Society of Anesthesiologists score>3), bleeding tendency due to heart disease or liver cirrhosis, renal tumors in patients with other advanced cancer, and very small tumors (<2 cm).
After kidney mobilization and perirenal tissue dissection, we performed intraoperative renal biopsy in every patient. Up to two needle biopsies were taken from the renal tumor before the insertion of a cryoneedle.
We performed computed tomography (CT) or magnetic resonance imaging (MRI) to evaluate residual tumors about 1 month after the operation. The patients were then evaluated every 3 months during the first year. After the first year, they were evaluated every 6 months during the follow-up period. The evaluations included laboratory tests, chest radiography, and CT or MRI.
We analyzed the patients' characteristics and perioperative outcomes. Local recurrence was defined as increasing tumor size or increasing enhancement of the tumor on imaging study. In our study, assessment of functional outcomes of RC was based on postoperative changes in the estimated glomerular filtration rate (eGFR), which was calculated by using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) [12].
The preoperative eGFR was compared with 1-month postoperative eGFR and 1-year postoperative eGFR by use of paired t-tests. We also performed logistic regression analysis by using SPSS and the "FIRTH" method with SAS 9.2 (SAS Institute Inc., Cary, NC, USA) to investigate the factors affecting local recurrence. All statistical analyses were performed by using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA), with statistical significance considered at p<0.05.
Of the 68 patients with RC who were treated in our institution, the number of treated renal tumors was 79. Among the total 68 patients, an initial 9 patients (13.2%) were treated by open surgery and the remaining 59 patients (86.8%) were treated by laparoscopic surgery. The patients' characteristics and perioperative outcomes are summarized in Table 1.
The mean age of the patients was 62 years (range, 22-81 years). The mean tumor size was 2.25 cm (range, 0.3-5.7 cm), and the mean RENAL score was 7 (range, 4-11). The mean operative time was 194 minutes (range, 85-290 minutes) and the mean estimated blood loss (EBL) was 75 mL (range, 0-250 mL). In our study, 1 patient (1.47%) was converted to open surgery, but no other intraoperative complications such as nephrectomy occurred.
Few postoperative complications occurred in our study. Only 3 patients (4.4%) had Clavien-Dindo grade II complications. Furthermore, no Clavien grade III, IV, or V (major) complications occurred during the postoperative period.
The mean preoperative and 1-month postoperative eGFR were 71.8 mL/min/1.73 m2 (range, 60.1-81.6 mL/min/1.73 m2) and 68.3 mL/min/1.73 m2 (range, 58.3-79.2 mL/min/1.73 m2), respectively (p=0.19). The mean 1-year postoperative eGFR was 65.0 mL/min/1.73 m2 (range, 55.3-76.4 mL/min/1.73 m2) (p=0.25). The 1-year postoperative eGFR was investigated in patients who had been followed up for more than 1 year.
The indications for RC in our institution are summarized in Tables 2 and 3. The absolute indications included renal tumors in a solitary kidney in 12 patients (17.65%), renal tumors with CKD in 6 patients (8.82%), bilateral renal tumors in 5 patients (7.35%), ipsilateral multiple renal tumors in 1 patient (1.47%), and Von Hippel Lindau disease in 2 patients (2.94%) (Fig. 1). All 6 patients who had CKD fell under CKD stage II (eGFR, 60-79). A total of 26 patients (38.23%) had absolute indications. The elective indications included age (older than 70 years) in 17 patients (25.00%), multiple comorbidities in 14 patients (20.59%), bleeding tendency in 6 patients (8.82%), renal tumors in patients with other advanced cancer in 15 patients (22.06%), and very small tumors in 10 patients (14.71%).
The pathologic findings are summarized in Table 4. In the pathologic examination, the total number of patients diagnosed with RCC was 52 (65.8%). All patients with RCC showed common clear cell carcinoma, except for 6 patients (7.6%) with the papillary type and 1 patient (1.3%) with the chromophobe type. The other malignancies included transitional cell carcinoma in 1 patient (1.3%) and tubulocystic carcinoma in 1 patient (1.3%). The benign pathologic f indings included angiomyolipoma in 13 patients (16.5%), oncocytoma in 1 patient (1.3%), fibroadipose tissue in 5 patients (6.3%), calcified cysts in 2 patients (2.5%), and chronic inflammation in 4 patients (5.0%).
The mean follow-up period was 59.76 months (range, 3-119 months). During the follow-up period, local recurrence was found in 8 tumors (15.4%, a total of 52 RCCs) with imaging studies. The mean interval from the time of surgery to the time of recurrence was 6.5 months (range, 2-9 months). The characteristics of the recurred tumors are summarized in Table 5.
We investigated on the basis of tumor size with a cutoff of 4 cm. Among 74 tumors smaller than 4 cm, RCC was confirmed in 48 tumors (64.8%) and local recurrence was found in 4 tumors (8.3%). Among 5 tumors greater than 4 cm, RCC was confirmed in 4 tumors (80.0%) and local recurrence was found in all 4 (100.0%). Logistic regression analysis was performed to investigate the factors affecting local recurrence. Among all factors, the results showed statistical significance only for the size of the tumor (p=0.006). The other factors did not affect local recurrence (Table 6).
Concerning treatment in patients with local recurrence, one patient underwent repeat cryosurgery and four patients underwent ultrasonography-guided radiofrequency ablation (Fig. 2). None of the five patients who underwent re-treatment for local recurrence experienced additional recurrence during the follow-up period. The remaining three patients are currently under active surveillance. Likewise, the active surveillance group did not experience tumor progression during the follow-up period.
In our study, distant metastasis was found in only 1 patient (1.47%). The patient underwent LRC to manage RCC. One year after surgery, an approximately 1.3-cm-sized pancreatic mass was identified by MRI. Consequently, the patient underwent additional surgery and the pancreatic mass was pathologically diagnosed as RCC.
In our study, the recurrence-free rate was 83.0% and the cancer-specific survival rate was 100%. Moreover, the 5- and 10-year overall survival rates were both 100%.
We are currently facing an increasing number of SRMs with undefined malignant potential. SRMs are more frequently detected in elderly patients with many comorbidities [13]. PN is the gold standard of treatment for managing SRMs [3]. However, PN is difficult to perform in elderly patients with comorbidities because of their poor operative status. More recently, active surveillance and ablative technologies have emerged as potential alternatives to surgery in selected patients. The American Urological Association guidelines consider patients with significant comorbidities who are unfit for PN as potential candidates for ablative technologies or for active surveillance in those wishing to avoid treatment [3]. In our study, we performed oncologic follow-up for a mean period of 59.76 months (range, 3-119 months) on a series of 68 patients who were treated with RC. Of the 68 patients, 26 patients (38.23%) had absolute indications for RC. The remaining patients had elective indications including multiple comorbidities and very small tumors (less than 2 cm).
Recently, many studies comparing the perioperative outcome of PN and RC have been reported. Klatte et al. [14] reported a systematic review comparing the perioperative and oncologic outcomes of LPN and LRC. In that study, LRC was associated with significantly shorter operative times, lower EBL, shorter hospital stay, and a lower risk of total complications compared with LPN. Also, the favorable morbidity of RC has been well established. Most published studies have reported low rates of major complications ranging from 1% to 6% [15,16,17]. More recently, multi-institutional data on LRC in 144 patients showed a total complication rate of 15.5%, and most complications were minor, such as pain [18].
The results of our study revealed a mean EBL of 75 mL (range, 0-250 mL) and a mean operative time of 194 minutes (range, 85-290 minutes). The mean hospital stay was 6.7 days (range, 3-13 days) and no patient experienced clinical signs or symptoms of collecting system injury. During the intraoperative period, only 1 case (1.47%) was converted to open surgery. Except for this, no perioperative complications occurred in any of our patients. Because of preoperative anemia, a total of 3 patients (4.4%) needed postoperative transfusion.
Several studies have reported functional outcomes of LRC. Park et al. [19] reported no significant change in renal function when pre-LRC Scr was compared with post-LRC Scr (7 days after cryoablation). Aron et al. [20] reported that LRC had a minimal impact on renal function. In that study, pre-LRC eGFR and post-LRC eGFR at a median of 24 months were 66 and 59 mL/min/1.73 m2, respectively.
In our study, we used eGFR to investigate functional outcomes of RC. Statistical comparison of preoperative and postoperative levels was performed with paired t-tests. The mean preoperative and 1-month postoperative eGFR were 71.8 mL/min/1.73 m2 (range, 60.1-81.6 mL/min/1.73 m2) and 68.3 mL/min/1.73 m2 (range, 58.3-79.2 mL/min/1.73 m2), respectively (p=0.19). The mean 1-year postoperative eGFR was 65.0 mL/min/1.73 m2 (range, 55.3-76.4 mL/min/1.73 m2) (p=0.25). The 1-year postoperative eGFR was investigated in patients who had been followed up for more than 1 year. As time passed, the mean eGFR did decrease, but this result was not statistically significant.
Recently, several studies with intermediate and long-term outcomes of LRC have been reported. Hegarty et al. [21] reported 5-year outcomes of 60 patients who were treated with LRC. Overall and cancer-specific survival rates were 82% and 100%, respectively, with local recurrence occurring in 3 patients (6.7%). Guillotreau et al. [22] reported outcomes of robotic PN and LRC in the treatment of patients with SRMs (≤4 cm). In that study, the local recurrence rate for LRC was 11%. Strom et al. [23] reported recurrence rates after percutaneous RC and LRC of SRMs. Local recurrence rates for percutaneous RC and LRC were 16.4% and 5.9%, respectively. The overall survival rates of percutaneous RC and LRC were 88.9% and 89.3%, respectively. However, compared with PN, most reported studies showed that the local recurrence of RC was higher than that of PN. Klatte et al. [14] reported a systematic review comparing the oncologic outcomes of LPN and LRC. In that study, patients who had undergone LRC had a significantly increased risk of local (relative risk [RR], 9.39) and systemic (RR, 4.68) tumor progression compared with patients who had undergone LPN.
In our study, local recurrence was found in 8 tumors (15.4%). Compared with other studies, the recurrence rate in our study was relatively higher. However, this is most likely because our study included the results of surgery on tumors greater than 4 cm. In practice, among a total of 74 tumors that were less than 4 cm, local recurrence occurred in 4 tumors (5.41%). Similar to other studies, the recurrence rate of tumors less than 4 cm was favorable in our study as well. Our results showed a higher recurrence rate for RC than for PN. However, there was no additional recurrence during the follow-up period after the patients underwent additional nephron-sparing treatment. In our study, the recurrence-free rate was 83.0% and the metastasis-free rate was 97.9%. Also, the 5- and 10-year overall survival rates were both 100%. Similar to previous reported studies [21], the oncologic outcomes of our study were favorable.
Recently, PN is increasingly being performed for T1b tumors (larger than 4 cm). Volpe et al. [24] reported outcomes of PN for T1b tumors. In that study, PN of T1b tumors achieved similar oncologic and even better renal functional outcomes than did radical nephrectomy. By contrast, in our study, the results of RC of T1b tumors were doubtful. Among the 5 tumors greater than 4 cm, local recurrence was found in 4 tumors (100.0%). The local recurrence rate was relatively higher in the case of tumors larger than 4 cm. Therefore, application of RC for tumors greater than 4 cm seems inappropriate.
Regarding the high recurrence rate of RC, several studies of factors that may contribute to local recurrence have been published. Tsivian et al. [25] reported the results of a regression analysis of risk factors for local recurrence after LRC. In the proportional hazards regression, tumor size (p=0.003; odds ratio [OR], 4.1) and endophytic growth pattern (p=0.28; OR,11.4) were significantly associated with local recurrence.
In our study, logistic regression analysis was performed to investigate the factors affecting local recurrence. Our results showed statistical significance only for the size of the tumor (p=0.005; OR, 3.8). An endophytic growth pattern had borderline significance in our study (p=0.081; OR, 14.398) (Table 6). Although it did not show statistical significance, an endophytic growth pattern tended to be associated with higher recurrence as the number of patients increased.
Therefore, on the basis of the results of our study, in cases in which PN is difficult owing to multiple comorbidities or in which the renal mass is less than 4 cm, RC is recommended. However, RC must be used cautiously if the renal mass has an endophytic pattern.
We recognize several limitations of this study. First, this study was based on the experience of a single institution with a relatively small number of patients. Second, it was based on a retrospective, noncomparative study via chart review. Therefore, to establish the effectiveness of RC, randomized and prospective studies with comparison to PN must be conducted. Although several intermediate- and long-term follow-up results of RC have been reported in Western countries, until now, few studies of functional and oncologic outcomes of RC had been reported in Korea. The strength of this study is that the research was based on a relatively long-term follow-up period in Korea. Moreover, we performed RC for patients who were selected by elective and absolute indications in the preoperative period. Similar to other studies, our results for RC were favorable.
In our study with long-term follow-up, RC for SRMs showed favorable perioperative, functional, and oncological outcomes without major complications. Compared to PN, RC was associated with a high recurrence rate. However, RC showed excellent oncologic outcomes after other nephron-sparing treatment was performed in the patients with local recurrence. If RC is performed on the basis of appropriate patient selection, it is expected to show effective results.
Figures and Tables
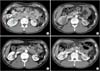 | Fig. 1Serial changes of the Von Hippel Lindau (VHL) disease patient. The patient underwent open left partial nephrectomy due to left renal mass on December 2010. (A) The preoperative computed tomography (CT) image showed a 1.7-cm endophytic tumor in right kidney midportion (arrow). (B) The postoperative CT image at 2 weeks. (C) The postoperative CT image at 3 months. The images showed a improved previous cryoablation site in right kidney. (D) The postoperative CT image at 1 year. |
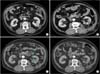 | Fig. 2Serial changes of a left renal tumor in local recurrence patient. (A) The preoperative computed tomography (CT) image showed a 3.7-cm tumor mass in posterior aspect of left kidney midportion. (B) The postoperative CT image at 3 months. (C) The postoperative CT image showed a recurred tumor in medial aspect of cryoablated lesion in left kidney (arrow). (D) The postradiofrequency ablation CT image at 2 months. |
Table 1
Patient characteristics and perioperative outcomes

Table 2
Absolute indications for renal cryoablation

| Indication | No. (%) |
|---|---|
| Solitary kidney | 12 (17.65) |
| Bilateral renal tumor | 5 (7.35) |
| Ipsilateral multiple tumor | 1 (1.47) |
| Hereditary renal tumor | 2 (2.94) |
| Chronic kidney disease | 6 (8.82) |
| Total | 26 (38.23) |
Table 3
Elective indications for renal cryoablation
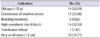
Table 4
Histopathological findings of renal cryoablation
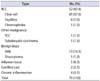
Table 5
Characteristics of recurred tumors in patients with biopsy-proven RCC

Table 6
Univariable and multivariable logistic regression analysis of the factors affecting local recurrence

ACKNOWLEDGMENTS
This study was supported by a research grant from the Korea University Medical College (Seoul, Korea).
References
1. Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014; 46:109–123.
2. Miller DC, Ruterbusch J, Colt JS, Davis FG, Linehan WM, Chow WH, et al. Contemporary clinical epidemiology of renal cell carcinoma: insight from a population based case-control study. J Urol. 2010; 184:2254–2258.
3. Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009; 182:1271–1279.
4. Kim SP, Shah ND, Weight CJ, Borah BJ, Han LC, et al. The relationship of postoperative complications with in-hospital outcomes and costs after renal surgery for kidney cancer. BJU Int. 2013; 111:580–588.
5. Klatte T, Grubmüller B, Waldert M, Weibl P, Remzi M. Laparoscopic cryoablation versus partial nephrectomy for the treatment of small renal masses: systematic review and cumulative analysis of observational studies. Eur Urol. 2011; 60:435–443.
6. Frank I, Colombo JR Jr, Rubinstein M, Desai M, Kaouk J, Gill IS. Laparoscopic partial nephrectomy for centrally located renal tumors. J Urol. 2006; 175(3 Pt 1):849–852.
7. Venkatesh R, Weld K, Ames CD, Figenshau SR, Sundaram CP, Andriole GL, et al. Laparoscopic partial nephrectomy for renal masses: effect of tumor location. Urology. 2006; 67:1169–1174.
8. Reisiger K, Venkatesh R, Figenshau RS, Bae KT, Landman J. Complex laparoscopic partial nephrectomy for renal hilar tumors. Urology. 2005; 65:888–891.
9. Lehman DS, Landman J. Kidney cancer ablative therapy: indications and patient selection. Curr Urol Rep. 2008; 9:34–43.
10. Schiffman M, Moshfegh A, Talenfeld A, Del Pizzo JJ. Laparoscopic renal cryoablation. Semin Intervent Radiol. 2014; 31:64–69.
11. Ljungberg B, Gudmundsson E, Christensen S, Lundstam S. Swedish Kidney Cancer Quality Register Group. Practice patterns for the surgical treatment of T1 renal cell carcinoma: a nationwide population-based register study. Scand J Urol. 2014; 48:445–452.
12. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604–612.
13. Jewett MA, Mattar K, Basiuk J, Morash CG, Pautler SE, Siemens DR, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol. 2011; 60:39–44.
14. Klatte T, Shariat SF, Remzi M. Systematic review and meta-analysis of perioperative and oncologic outcomes of laparoscopic cryoablation versus laparoscopic partial nephrectomy for the treatment of small renal tumors. J Urol. 2014; 191:1209–1217.
15. Desai MM, Aron M, Gill IS. Laparoscopic partial nephrectomy versus laparoscopic cryoablation for the small renal tumor. Urology. 2005; 66:5 Suppl. 23–28.
16. O'Malley RL, Berger AD, Kanofsky JA, Phillips CK, Stifelman M, Taneja SS. A matched-cohort comparison of laparoscopic cryoablation and laparoscopic partial nephrectomy for treating renal masses. BJU Int. 2007; 99:395–398.
17. Johnson DB, Solomon SB, Su LM, Matsumoto ED, Kavoussi LR, Nakada SY, et al. Defining the complications of cryoablation and radio frequency ablation of small renal tumors: a multi-institutional review. J Urol. 2004; 172:874–877.
18. Laguna MP, Beemster P, Kumar V, Klingler HC, Wyler S, Anderson C, et al. Perioperative morbidity of laparoscopic cryoablation of small renal masses with ultrathin probes: a European multicentre experience. Eur Urol. 2009; 56:355–361.
19. Park SH, Kang SH, Ko YH, Kang SG, Park HS, Moon du G, et al. Cryoablation for endophytic renal cell carcinoma: intermediate-term oncologic efficacy and safety. Korean J Urol. 2010; 51:518–524.
20. Aron M, Kamoi K, Remer E, Berger A, Desai M, Gill I. Laparoscopic renal cryoablation: 8-year, single surgeon outcomes. J Urol. 2010; 183:889–895.
21. Hegarty NJ, Gill IS, Desai MM, Remer EM, O'Malley CM, Kaouk JH. Probe-ablative nephron-sparing surgery: cryoablation versus radiofrequency ablation. Urology. 2006; 68:1 Suppl. 7–13.
22. Guillotreau J, Haber GP, Autorino R, Miocinovic R, Hillyer S, Hernandez A, et al. Robotic partial nephrectomy versus laparoscopic cryoablation for the small renal mass. Eur Urol. 2012; 61:899–904.
23. Strom KH, Derweesh I, Stroup SP, Malcolm JB, L'Esperance J, Wake RW, et al. Second prize: Recurrence rates after percutaneous and laparoscopic renal cryoablation of small renal masses: does the approach make a difference? J Endourol. 2011; 25:371–375.
24. Volpe A, Amparore D, Mottrie A. Treatment outcomes of partial nephrectomy for T1b tumours. Curr Opin Urol. 2013; 23:403–410.
25. Tsivian M, Lyne JC, Mayes JM, Mouraviev V, Kimura M, Polascik TJ. Tumor size and endophytic growth pattern affect recurrence rates after laparoscopic renal cryoablation. Urology. 2010; 75:307–310.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download