Abstract
This article summarizes anatomical, neurophysiological, and pharmacological studies in humans and animals to provide insights into the neural circuitry and neurotransmitter mechanisms controlling the lower urinary tract and alterations in these mechanisms in lower urinary tract dysfunction. The functions of the lower urinary tract, to store and periodically release urine, are dependent on the activity of smooth and striated muscles in the bladder, urethra, and external urethral sphincter. During urine storage, the outlet is closed and the bladder smooth muscle is quiescent. When bladder volume reaches the micturition threshold, activation of a micturition center in the dorsolateral pons (the pontine micturition center) induces a bladder contraction and a reciprocal relaxation of the urethra, leading to bladder emptying. During voiding, sacral parasympathetic (pelvic) nerves provide an excitatory input (cholinergic and purinergic) to the bladder and inhibitory input (nitrergic) to the urethra. These peripheral systems are integrated by excitatory and inhibitory regulation at the levels of the spinal cord and the brain. Therefore, injury or diseases of the nervous system, as well as disorders of the peripheral organs, can produce lower urinary tract dysfunction, leading to lower urinary tract symptoms, including both storage and voiding symptoms, and pelvic pain. Neuroplasticity underlying pathological changes in lower urinary tract function is discussed.
The storage and periodic elimination of urine depend on the coordinated activity of two functional units in the lower urinary tract (LUT): (1) a reservoir (the urinary bladder) and (2) an outlet consisting of the bladder neck, the urethra, and the urethral sphincter [1]. Coordination between these organs is mediated by a complex neural control system located in the brain, spinal cord, and peripheral ganglia [2]. Thus, urine storage and release are highly dependent on central nervous system pathways. This distinguishes the LUT from many other visceral structures (e.g., the gastrointestinal tract and cardiovascular system) that maintain a certain level of function even after extrinsic neural input has been eliminated.
The LUT is also unusual in its pattern of activity and organization of neural control mechanisms. For example, the urinary bladder has only two modes of operation: storage and elimination. Thus, many of the neural circuits have switch-like or phasic patterns of activity [3,4], unlike the tonic patterns characteristic of the autonomic pathways to cardiovascular organs. Micturition also requires the integration of autonomic and somatic efferent mechanisms to coordinate the activity of visceral organs (the bladder and urethra) with that of urethral striated muscles [2,4].
Owing to the complexity of the neural mechanisms regulating the LUT, micturition is sensitive to a wide variety of injuries, diseases, and chemicals that affect the nervous system. Thus, neurologic mechanisms are an important consideration in the diagnosis and treatment of voiding disorders. This article reviews (1) the innervation of the LUT, (2) the organization of the reflex pathways controlling urine storage and elimination, and (3) neurogenic dysfunctions of the LUT.
The reciprocal function of the bladder and urethra, i.e., storage and voiding, is peripherally coordinated by three sets of nerves, the parasympathetic, sympathetic, and somatic peripheral nerves, that are components of intricate efferent and afferent circuitry derived from the brain and the spinal cord [5,6] (Figs. 1, 2). These nerves also carry sensory information in afferent fibers from the LUT to the lumbosacral spinal cord, which is composed of myelinated Aδ-fibers and unmyelinated C-fibers [6-10] (Fig. 3). Aδ-Fibers, which are located primarily within the detrusor smooth muscle layer, respond primarily to detrusor stretching during bladder filling and convey sensations of fullness. Unmyelinated sensory C-fibers are more widespread and reside in the muscle, close to the urothelium in the mucosa and directly adjacent to the urothelial cells themselves [6,11,12].
Until the volume of urine in the bladder reaches a critical threshold for voiding, the detrusor is quiet, the bladder having low and relatively constant levels of internal pressure during filling [6]. During this phase, the intrinsic viscoelasticity of detrusor muscles permits the bladder wall to adjust to increasing volume by stretching and the stimulatory parasympathetic pathway is quiescent. However, there are also neurogenic contributions toward maintaining an inactive bladder during the storage phase [6,13-15] (Fig. 4).
The bladder-to-external urethral sphincter (EUS) reflex, the guarding reflex, is initiated by distension of the bladder during filling, which activates stretch-sensitive mechanoceptors in the bladder wall, in turn generating afferent signals to the sacral spinal cord where pudendal motoneurons are activated. The pudendal nerve efferents stimulate EUS contractions, thereby maintaining outlet resistance and urinary continence. The guarding reflex increases in intensity as the bladder volume increases (Fig. 4).
The bladder-to-sympathetic pathway reflex is also triggered by bladder distension. Stimulated bladder afferents activate an intersegmental pathway from sacral cord to thoracolumbar sympathetic nerves. The activated sympathetic nerves stimulate contraction of the internal urethral sphincter and inhibit bladder activity [14] (Fig. 4).
The reflexes involved in urine storage are predominantly integrated in the spinal cord and seem to function normally in animals with supraspinal transections. However, voluntary control of micturition is impaired in many patients with lesions that interrupt brain stem pathways. Thus, although the storage reflex seems to be established within the spinal cord, maintaining a stable urethral resistance apparently requires supraspinal input [6,16]. It is known that the pontine urine storage center located in the dorsolateral pons provides descending inputs that activate pudendal motoneurons and thus increase urethral resistance (Fig. 4).
The essential first step in micturition is relaxation of the EUS muscles. In adult humans with normal LUT function, the individual has sensory awareness of a full bladder, and the guarding reflex is intensified until voluntary elimination is possible. Initiation as well as normal completion of the elimination process depends on input from the brain [6,16] (Fig. 5).
In order for the guarding reflex to be reversed and the EUS relaxed, a final inhibitory signal is generated from the pontine micturition center (PMC). Bladder afferent fibers in the pelvic nerve form synapses in the spinal cord, and axons from the second-order neurons travel rostrally to the micturition center. The center integrates this sensory information with signals from more rostral brain regions and ultimately generates inhibitory input to the sympathetic and somatic centers in the spinal cord and stimulatory input to the parasympathetic center (Fig. 5). This spino-bulbo-spinal reflex results in relaxation of the EUS and internal urethral sphincter, followed by contraction of detrusor muscles, increase in bladder pressure, and flow of urine [4].
Spinal cord injury (SCI) rostral to the lumbosacral level eliminates voluntary and supraspinal control of voiding, leading initially to an areflexic bladder and complete urinary retention followed by a slow development of automatic micturition and bladder overactivity mediated by spinal reflex pathways [17-24]. However, voiding is commonly inefficient owing to simultaneous contractions of the bladder and urethral sphincter (detrusor-sphincter-dyssynergia, or DSD). Electrophysiologic studies in animals have shown that the micturition reflex pathways in spinal-intact animals and in chronic spinal-injured animals are markedly different [25,26]. In cats with an intact spinal cord, myelinated Aδ afferents activate the micturition reflex, whereas in cats with chronic thoracic spinal cord transection, micturition is induced by unmyelinated C-fiber axons. In normal cats, capsaicin does not block reflex contractions of the bladder or the Aδ-fiber-evoked bladder reflex. However, in cats with chronic spinal injury, capsaicin, a neurotoxin known to disrupt the function of C-fiber afferents, completely blocks C-fiber-evoked bladder reflexes [19,26]. Fig. 6 depicts the hypothetical mechanisms inducing lower urinary tract symptoms (LUTS) and bladder overactivity following bladder dysfunction induced by pathological conditions including SCI.
Chronic SCI in humans also causes the emergence of an unusual bladder reflex that is elicited in response to infusion of cold water into the bladder [27]. Studies in animals indicate that cold temperature activates receptors in bladder C-fiber afferents and urothelial cells [28,29]. Contribution of afferent hyperexcitability to the emergence of bladder overactivity in SCI has also been identified by clinical studies using neurotoxins such as botulinum toxin and resiniferatoxin (RTX). For example, suppression of bladder afferent activity with botulinum toxin effectively treats detrusor overactivity (DO), mitigates urgency in both neurogenic DO in SCI patients, and, with sustained therapy, reduces the expression of the capsaicin receptor (TRPV1) and the purinergic receptor in suburothelial nerve fibers [30,31]. In patients with SCI-induced DO, the clinical response to intravesical therapy with the C-fiber afferent toxin RTX is accompanied by a marked decrease in the density of nerve fibers staining positively for PGP9.5 and TRPV1 [32,33].
The emergence of C-fiber bladder reflexes seems to be mediated by several mechanisms, including changes in central synaptic connections and alterations in the properties of the peripheral afferent receptors that lead to sensitization of the 'silent' C fibers and the unmasking of responses to mechanical stimuli [22-24,34] (Fig. 6). In rats, it has been shown that bladder afferent neurons undergo both morphologic (neuronal hypertrophy) [35] and physiologic changes, including a shift from the high-threshold, tetrodotoxin (TTX)-resistant Na+ channel type to the low-threshold, TTX-sensitive Na+ channel type [21,36]. Other physiologic changes include the down-regulation of low-threshold A-type K+ channels, which is associated with decreased expression of Kv1.4 α-subunit following SCI [37].
Destruction of lumbosacral spinal neurons expressing neurokinin-1 (NK-1) receptors using substance P conjugated with saporin does not affect reflex voiding in normal rats, but reduces the bladder irritant effects of intravesical capsaicin [38] and reduces nonvoiding contractions in SCI rats [39]. Similarly, intrathecal administration of a selective NK-1 receptor antagonist (L-733060) does not affect the micturition reflex in spinal-intact rats but blocks the micturition reflex in SCI rats [40]. These data coupled with the increased expression of substance P in the region of the sacral parasympathetic nucleus in SCI rats [40] suggest that activation of NK-1 receptors in the spinal cord plays a role in SCI-induced DO.
Reduced γ-aminobutyric acid (GABA)-ergic inhibition could contribute to the development of neurogenic DO because mRNA levels of GAD67, an enzyme involved in GABA synthesis, are decreased in the spinal cord after SCI in rats [41]. A possible therapy for neurogenic DO has emerged from experimental studies, in which a herpes simplex virus vector encoding the GAD gene was injected into the bladder of SCI rats to increase GAD expression in bladder afferent nerves. This treatment reduces DO and DSD in SCI rats [42,43].
The number of suburothelial nerve fibers expressing TRPV1 receptors, which are predominantly expressed in C-fiber afferent pathways, is increased in patients with neurogenic DO compared to controls [32]. In rats with SCI, duodenal administration of a TRPV1 antagonist (GRC 6211) reduces bladder contraction frequency in SCI rats [44]. In addition, intravenous administration of a TRPA1 antagonist (HC-030031) or intrathecal treatment with antisense oligodeoxynucleotide of TRPA1 receptors is effective in suppressing DO in SCI rats [45]. These results along with the increased TRPA1 expression in the bladder and L6-S1 dorsal root ganglion (DRG) in SCI rats [45] suggest that TRPV1 and TRPA1 channels are involved in the emergence of C-fiber bladder afferent hyperexcitability that contributes to neurogenic DO in SCI.
It has been speculated that SCI-induced neuroplasticity is mediated by the actions of neurotrophic factors such as nerve growth factor (NGF) released within the urinary bladder or the spinal cord (Fig. 6). In clinical studies, NGF production is elevated in the bladder and in urine samples of SCI patients with DO and can be reduced along with symptom improvements after intradetrusor botulinum toxin treatment [46,47]. Animal studies have also demonstrated that the production of neurotrophic factors including NGF increases in the bladder after SCI [48]. Furthermore, chronic administration of NGF into the spinal cord or into the bladder wall in rats induces bladder overactivity and increased excitability of bladder afferent neurons [49-51]. Increased transport of NGF to DRG cell bodies or central NGF production in the injured spinal cord could modulate the micturition pathway at the spinal level. Intrathecal delivery of an NGF monoclonal antibody diminishes neurogenic DO and DSD in rats with SCI [39,52]. Thus, a combination of peripheral and central NGF actions is likely to be involved in the emergence of neurogenic DO.
Bladder outlet obstruction (BOO), which can occur in patients with benign prostatic hyperplasia, often produces detrusor hypertrophy and DO [5,53].
BOO alters neural networks in the central nervous system to induce bladder dysfunction (Fig. 6). BOO in rats enhances a spinal micturition reflex [54] and clinically enhances the bladder ice water test, which is mediated by a C-fiber afferent-dependent spinal micturition reflex. This finding in rats is consistent with a considerable upregulation of C-fiber afferent mechanisms in BOO patients with benign prostatic hyperplasia [55-57]. Within the spinal cord, obstruction stimulates an increased expression of GAP-43, an effect that is often associated with axonal sprouting after injury [58]. These observations suggest an enhancement or de novo development of new spinal circuits after obstruction.
Increased myogenic activity of detrusor smooth muscles is another important mechanism inducing overactive bladder and DO, which seems to be more applicable to patients with BOO. Partial BOO increases intravesical pressure and induces bladder hypertrophy and partial denervation of the bladder smooth muscle, leading to various functional changes in smooth muscles. These changes include denervation supersensitivity of cholinergic (muscarinic) receptors [59], increases in purinergic receptor-mediated contractile responses as well as expression of purinergic receptors such as P2X1 [60,61], and changes in the cell-to-cell communication in detrusor muscles owing to upregulation of gap-junction proteins such as connexin 43 [62,63]. In addition, another population of cells in the bladder known as interstitial cells has been proposed for a pacemaking role in spontaneous activity of the bladder [6,10]. It has been reported that the number of interstitial cells is increased in guinea pig and rat models of BOO [64,65] and that c-kit tyrosine kinase inhibitors, which inhibit interstitial cell activity, decrease the amplitude of spontaneous contractions in the guinea pig and human bladder [66,67]. These findings suggest that interstitial cells may also be involved in the emergence of DO as the result of enhanced autonomous detrusor muscle activity.
Men with overactive bladder symptoms and BOO caused by benign prostatic hyperplasia display increased levels of NGF in bladder tissues [68] and increased levels of urinary NGF [69]. NGF content is also increased in obstructed bladders in BOO animals [68,70]. Moreover, blockade of NGF action using autoantibodies prevents the neural plasticity and urinary frequency after obstruction and, in rats with persistent urinary frequency after relief of obstruction, NGF remains elevated in the bladder [68]. These findings suggest a cause-and-effect relationship between NGF-mediated changes in bladder afferents and an enhanced spinal micturition reflex and urinary frequency associated with BOO.
Bladder pain syndrome/interstitial cystitis (BPS/IC) is a disease with LUTS such as urinary frequency with bladder pain related to bladder filling. Although the etiology of BPS/IC is still not known, increasing evidence suggests that the disorder is associated with urothelial dysfunction and afferent hyperexcitability due to neurogenic bladder inflammation [71-73] (Fig. 6). In a rat model of chronic cystitis induced by cyclophosphamide or hydrochloric acid, it was shown that capsaicin-sensitive bladder afferent neurons increase their excitability owing to decreased density of A-type K+ (KA) currents, associated with the decreased expression of the Kv1.4 α-subunit [74,75]. Increased afferent hyperexcitability in BPS/IC is also supported by previous clinical findings that C-fiber desensitization induced by intravesical application of high-dose capsaicin or RTX is effective for treating painful symptoms in patients with IC [76,77]. However, a recent prospective, randomized clinical trial using intravesical RTX application failed to show a significant improvement of symptoms in patients with IC [78].
In patients with BPS/IC, there is a significant increase in suburothelial nerve fibers expressing TRPV1 and the increase is correlated with pain scores [79]. There is also evidence that chronic bladder inflammation in animal models can induce changes in functional properties of chemosensitive receptors such as TRPV1 in sensory neurons. In rat bladders, increased expression of anandamide, which can activate TRPV1 receptors, has been proposed as one mechanism that could contribute to the bladder overactivity elicited by cyclophosphamide-induced cystitis [80]. In addition, bladder overactivity elicited in rats by lipopolysaccharide-induced cystitis is inhibited by intraduodenal administration of a TRPV1 antagonist (GRC-6211) [81] and is prevented in TRPV1-knockout mice [82]. Therefore, it is assumed that enhanced activity of TRPV1 receptors in bladder afferent pathways contributes to bladder pain in BPS/IC.
In patients with BPS/IC, increased levels of neurotrophic factors, including NGF, neurotrophin-3, and glial-derived neurotrophic factor, have been detected in the urine [83]. Increased expression of NGF is also present in bladder biopsies from women with BPS/IC [84]. It has also been demonstrated that exogenous NGF can induce bladder nociceptive responses and bladder overactivity in rats when applied acutely into the bladder lumen [85,86] or chronically to the bladder wall or intrathecal space [49,50]. Thus, target organ-neural interactions mediated by an increase of neurotrophic factors in the bladder and increased transport of neurotrophic factors to the neuronal cell bodies in afferent pathways may contribute to the emergence of bladder pain and other symptoms, such as urinary frequency, in BPS/IC.
In clinical studies, the monoclonal NGF neutralizing antibody, tanezumab, has been tested, and encouraging results of the Phase II efficacy study were obtained [87]. Although proof-of-concept evidence has been provided for the effectiveness of systemic targeting of the NGF system in the treatment of BPS/IC, clinical studies were put on hold following reports of bone necrosis requiring total joint replacements in clinical trials for osteoarthritis (www.clinicaltrials.gov). Therefore, site-specific reduction of NGF would be desirable to reduce the intrinsic toxicity from systemic blockade of NGF. In this regard, a recent study using rats showed that treatment with intravesical liposomal antisense suppresses NGF expression in the urothelium as well as bladder overactivity and chemokine upregulation in a model of acetic acid-induced bladder overactivity [88]. Thus, local suppression of NGF in the bladder using intravesical liposome-based delivery techniques could be an attractive approach for BPS/IC treatment. Such an approach could avoid the systemic side effects that may be associated with nonspecific blockade of NGF expression.
A large percentage (50%-70%) of patients with diabetes mellitus (DM) exhibit LUTS [89,90]. The most common urodynamic findings, classically referred to as diabetic cystopathy, include impaired sensation of bladder fullness, increased bladder capacity, reduced bladder contractility, and increased residual urine [90-92]. However, DO is also common in patients with DM, especially when they present with LUTS [89,93].
The pathophysiology of DM-associated LUT complications is multifactorial and can be a result of an alteration in the physiology of the detrusor smooth muscle cell, the peripheral innervation reflex mechanisms, or urothelial function [92]. A two-step model of diabetic cystopathy progression has been proposed on the basis of experimental animal studies. This model suggests that patients initially develop bladder hypertrophy and overactivity, which is presumably a process of adaptation to polyuria-mediated frequent voiding, followed by the decompensated phase inducing classical diabetic cystopathy associated with detrusor underactivity [94]. DM neuropathy also reportedly affects urethral function in streptozotocin-induced DM rats. DM rats exhibit a reduction in nitric oxide-mediated relaxation and an enhancement of α1-adrenoceptor-mediated contraction of the urethral smooth muscle during reflex bladder contractions, both of which may contribute to increased bladder outlet resistance resulting in impaired bladder emptying in DM [95,96].
A two-step model of diabetic cystopathy progression (i.e., initial overactivity followed by underactivity) is also supported by changes in NGF levels in the bladder and axonal transport of NGF. Increased NGF levels have been reported in the bladders of rats with early experimental DM [97], whereas in more advanced diabetic bladder disease (for example, the decompensated phase of experimental diabetic cystopathy), bladder and DRG levels of NGF drop and animals display increased bladder capacity and decreased peak pressures [98]. Loss of NGF production has been implicated in the development of sensory and sympathetic neuronal degeneration associated with diabetic neuropathy [99,100].
Storage and periodic release of urine is dependent on a complex neural network located at various levels of the peripheral and central nervous system that coordinates the activity of smooth and striated muscles of the bladder and urethra. Afferent pathways that trigger storage and voiding reflexes as well as the sensations of bladder filling transmit activity from mechanoreceptors in the bladder through second order neurons in the spinal cord to various central processing areas in the brain.
Owing to the complexity of the neural mechanisms that regulate urine storage and voiding, these processes are sensitive to various neural injuries and diseases. As the underlying mechanism inducing LUT dysfunction, several types of peripheral and central neuroplasticity have been identified. These include the following: (1) emergence of primitive neonatal micturition reflexes and (2) remodeling of spinal circuitry and sensitization of bladder silent C-fiber afferents leading to the emergence of a spinal micturition reflex. NGF has been implicated in this plasticity because NGF levels increase in the bladder and spinal cord. Furthermore, intrathecal administration of NGF antibodies suppresses neurogenic LUT dysfunction and afferent sensitization in animal models. Increased levels of neurotrophic factors have also been detected in other types of bladder disorders. Therefore, the role of these agents in bladder pathophysiological mechanisms is a very active research field in neurourology.
Figures and Tables
 | FIG. 1Major preganglionic and postganglionic neural pathways from the spinal cord to the lower urinary tract. The sympathetic hypogastric nerve, emerging from the inferior mesenteric ganglion, stimulates urethral smooth muscle. The parasympathetic pelvic nerve, making synapses onto postganglionic neurons in the pelvic ganglion, stimulates bladder detrusor muscle and inhibits urethral smooth muscle. The somatic pudendal nerve stimulates striated muscle of the external urethral sphincter (EUS). ACh, acetylcholine; NE, norepinephrine; NO, nitric oxide; S2-S4, sacral segments of the spinal cord; T10-L2, thoracolumbar segments of the spinal cord. Adapted from Yoshimura et al. Naunyn Schmiedebergs Arch Pharmacol 2008;377:437-48 [4]. |
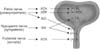 | FIG. 2Innervation of the lower urinary tract. The parasympathetic pelvic nerve stimulates the bladder detrusor muscle, mediated by muscarinic receptors (M3) being activated by acetylcholine (ACh) and purinergic receptors (P2X1) being activated by adenosine triphosphate (ATP), and relaxes the urethral smooth muscle, mediated by nitric oxide (NO). The sympathetic hypogastric nerve stimulates urethral smooth muscle and inhibits bladder detrusor, mediated by α1-adrenergic and β3-adrenergic receptors, respectively. The somatic pudendal nerve stimulates striated muscle of the external urethral sphincter, mediated by ACh activating nicotinic (N) receptors. NA, noradrenaline. Plus and minus signs in parentheses indicate neural stimulation and inhibition, respectively. |
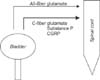 | FIG. 3Afferent fibers transmitting sensory information from the lower urinary tract to the spinal cord. Glutamate is a neurotransmitter present in both the Aδ- and C-fibers; additional neurotransmitters in the C fibers include substance P and calcitonin gene-related peptide (CGRP). Adapted from Yoshimura et al. Naunyn Schmiedebergs Arch Pharmacol 2008;377:437-48 [4]. |
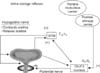 | FIG. 4Major reflex pathways to the lower urinary tract initiating and maintaining urine storage. α1, α-adrenergic receptors; β3, β-adrenergic receptors; N, nicotinic receptors; S2-S4, sacral segments of the spinal cord; T10-L2, thoracolumbar segments of the spinal cord; SYM, sympathetic nerve. Plus and minus signs indicate neural stimulation and inhibition, respectively. Adapted from Yoshimura et al. Naunyn Schmiedebergs Arch Pharmacol 2008;377:437-48 [4]. |
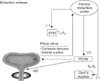 | FIG. 5Major reflex pathways from the pontine micturition center in the brainstem to the lower urinary tract via the spinal cord regulating micturition. M3, muscarinic receptors; S2-S4, sacral segments of the spinal cord; T10-L2, thoracolumbar segments of the spinal cord; SYM, sympathetic nerve; PSYM, parasympathetic nerve. Plus and minus signs indicate neural stimulation and inhibition, respectively. Descending inputs from the pontine micturition center inhibit neuronal activity in the sympathetic nervous system and Onuf's nuclei in the spinal cord. Adapted from Yoshimura et al. Naunyn Schmiedebergs Arch Pharmacol 2008;377:437-48 [4]. |
 | FIG. 6Diagram showing hypothetical mechanisms inducing bladder overactivity, LUTS, and pelvic pain. Bladder dysfunction (1) causes increased production of neurotrophic factors such as NGF or other chemical mediators in the bladder wall (2). NGF is taken up by afferent nerves and transported to DRG cells (3) where it changes gene expression, which leads to changes in functional properties of ion channels and receptors and increased neuronal excitability (4). Increased afferent nerve activity results in enhanced synaptic transmission in the spinal cord (5), leading to LUTS or pelvic pain (6) and bladder overactivity (7). BOO, bladder outlet obstruction; DRG, dorsal root ganglion; LUTS, lower urinary tract symptoms; NGF, nerve growth factor. |
ACKNOWLEDGMENTS
The authors' research is supported by NIH grants (P01DK093424, DK088836), DOD grants (W81XWH-11-1-0763, W81XWH-12-1-0565), and PVA 2793.
References
1. Fry C, Brading AF, Hussain M. Cell biology. In : Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Plymouth, UK: Health Publication Ltd.;2005. p. 313–362.
2. Morrison JF, Birder L, Craggs M. Neural control. In : Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Plymouth, UK: Health Publication Ltd.;2005. p. 363–422.
3. de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In : Maggi CA, editor. Nervous control of the urogenital system: autonomic nervous system. London: Harwood Academic Publishers;1993. p. 227–290.
4. Yoshimura N, Kaiho Y, Miyazato M, Yunoki T, Tai C, Chancellor MB, et al. Therapeutic receptor targets for lower urinary tract dysfunction. Naunyn Schmiedebergs Arch Pharmacol. 2008; 377:437–448.
5. Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004; 56:581–631.
6. Yoshimura N, Chancellor MB. Physiology and pharmacology of the bladder and urethra. In : Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh urology. 9th ed. Philadelphia: Saunders;2011. p. 1786–1833.
7. Morgan C, Nadelhaft I, de Groat WC. The distribution of visceral primary afferents from the pelvic nerve to Lissauer's tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol. 1981; 201:415–440.
8. Morgan C, deGroat WC, Nadelhaft I. The spinal distribution of sympathetic preganglionic and visceral primary afferent neurons that send axons into the hypogastric nerves of the cat. J Comp Neurol. 1986; 243:23–40.
9. Thor KB, Morgan C, Nadelhaft I, Houston M, De Groat WC. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol. 1989; 288:263–279.
10. Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004; 84:935–986.
11. Andersson KE. Bladder activation: afferent mechanisms. Urology. 2002; 59:5 Suppl 1. 43–50.
12. Ouslander JG. Management of overactive bladder. N Engl J Med. 2004; 350:786–799.
13. De Groat WC, Lalley PM. Reflex firing in the lumbar sympathetic outflow to activation of vesical afferent fibres. J Physiol. 1972; 226:289–309.
14. de Groat WC, Theobald RJ. Reflex activation of sympathetic pathways to vesical smooth muscle and parasympathetic ganglia by electrical stimulation of vesical afferents. J Physiol. 1976; 259:223–237.
15. Nishizawa O, Fukuda T, Matsuzaki A, Moriya I, Harada T, Tsuchida S. Role of the sympathetic nerve in bladder and urethral sphincter function during the micturition cycle in the dog evaluated by pressure flow EMG study. J Urol. 1985; 134:1259–1261.
16. Blaivas JG. The neurophysiology of micturition: a clinical study of 550 patients. J Urol. 1982; 127:958–963.
17. De Groat WC. Nervous control of the urinary bladder of the cat. Brain Res. 1975; 87:201–211.
18. de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981; 3:135–160.
19. de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, et al. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst. 1990; 30:Suppl. S71–S77.
20. Araki I, de Groat WC. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. J Neurosci. 1997; 17:8402–8407.
21. Yoshimura N. Bladder afferent pathway and spinal cord injury: possible mechanisms inducing hyperreflexia of the urinary bladder. Prog Neurobiol. 1999; 57:583–606.
22. de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006; 152:59–84.
23. de Groat WC, Yoshimura N. Changes in afferent activity after spinal cord injury. Neurourol Urodyn. 2010; 29:63–76.
24. de Groat WC, Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp Neurol. 2012; 235:123–132.
25. de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J Physiol. 1969; 200:87–108.
26. Cheng CL, Liu JC, Chang SY, Ma CP, de Groat WC. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am J Physiol. 1999; 277(3 Pt 2):R786–R794.
27. Geirsson G, Lindstrom S, Fall M. The bladder cooling reflex in man: characteristics and sensitivity to temperature. Br J Urol. 1993; 71:675–680.
28. Fall M, Lindstrom S, Mazieres L. A bladder-to-bladder cooling reflex in the cat. J Physiol. 1990; 427:281–300.
29. Stein RJ, Santos S, Nagatomi J, Hayashi Y, Minnery BS, Xavier M, et al. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J Urol. 2004; 172:1175–1178.
30. Apostolidis A, Brady CM, Yiangou Y, Davis J, Fowler CJ, Anand P. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology. 2005; 65:400–405.
31. Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005; 174:977–982.
32. Brady CM, Apostolidis A, Yiangou Y, Baecker PA, Ford AP, Freeman A, et al. P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur Urol. 2004; 46:247–253.
33. Brady CM, Apostolidis AN, Harper M, Yiangou Y, Beckett A, Jacques TS, et al. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int. 2004; 93:770–776.
34. de Groat WC. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Paraplegia. 1995; 33:493–505.
35. Kruse MN, Bray LA, de Groat WC. Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. J Auton Nerv Syst. 1995; 54:215–224.
36. Yoshimura N, de Groat WC. Plasticity of Na+ channels in afferent neurones innervating rat urinary bladder following spinal cord injury. J Physiol. 1997; 503(Pt 2):269–276.
37. Takahashi R, Yoshizawa T, Yunoki T, Tyagi P, Naito S, de Groat WC, et al. Hyperexcitability of bladder afferent neurons associated with reduction of Kv1.4 α-subunit in rats with spinal cord injury. J Urol. 2013; 190:2296–2304.
38. Seki S, Erickson KA, Seki M, Nishizawa O, Igawa Y, Ogawa T, et al. Elimination of rat spinal neurons expressing neurokinin 1 receptors reduces bladder overactivity and spinal c-fos expression induced by bladder irritation. Am J Physiol Renal Physiol. 2005; 288:F466–F473.
39. Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor MB, De Groat WC, et al. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J Urol. 2004; 171:478–482.
40. Zhang X, Douglas KL, Jin H, Eldaif BM, Nassar R, Fraser MO, et al. Sprouting of substance P-expressing primary afferent central terminals and spinal micturition reflex NK1 receptor dependence after spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2008; 295:R2084–R2096.
41. Miyazato M, Sasatomi K, Hiragata S, Sugaya K, Chancellor MB, de Groat WC, et al. GABA receptor activation in the lumbosacral spinal cord decreases detrusor overactivity in spinal cord injured rats. J Urol. 2008; 179:1178–1183.
42. Miyazato M, Sugaya K, Goins WF, Wolfe D, Goss JR, Chancellor MB, et al. Herpes simplex virus vector-mediated gene delivery of glutamic acid decarboxylase reduces detrusor overactivity in spinal cord-injured rats. Gene Ther. 2009; 16:660–668.
43. Miyazato M, Sugaya K, Saito S, Chancellor MB, Goins WF, Goss JR, et al. Suppression of detrusor-sphincter dyssynergia by herpes simplex virus vector mediated gene delivery of glutamic acid decarboxylase in spinal cord injured rats. J Urol. 2010; 184:1204–1210.
44. Santos-Silva A, Charrua A, Cruz CD, Gharat L, Avelino A, Cruz F. Rat detrusor overactivity induced by chronic spinalization can be abolished by a transient receptor potential vanilloid 1 (TRPV1) antagonist. Auton Neurosci. 2012; 166:35–38.
45. Andrade EL, Forner S, Bento AF, Leite DF, Dias MA, Leal PC, et al. TRPA1 receptor modulation attenuates bladder overactivity induced by spinal cord injury. Am J Physiol Renal Physiol. 2011; 300:F1223–F1234.
46. Giannantoni A, Di Stasi SM, Nardicchi V, Zucchi A, Macchioni L, Bini V, et al. Botulinum-A toxin injections into the detrusor muscle decrease nerve growth factor bladder tissue levels in patients with neurogenic detrusor overactivity. J Urol. 2006; 175:2341–2344.
47. Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-A injection. Eur Urol. 2009; 56:700–706.
48. Vizzard MA. Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury. Prog Brain Res. 2006; 152:97–115.
49. Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, et al. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci. 2006; 26:10847–10855.
50. Lamb K, Gebhart GF, Bielefeldt K. Increased nerve growth factor expression triggers bladder overactivity. J Pain. 2004; 5:150–156.
51. Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol. 2007; 7:9.
52. Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, et al. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol. 2002; 168:2269–2274.
53. Gosling JA, Kung LS, Dixon JS, Horan P, Whitbeck C, Levin RM. Correlation between the structure and function of the rabbit urinary bladder following partial outlet obstruction. J Urol. 2000; 163:1349–1356.
54. Steers WD, De Groat WC. Effect of bladder outlet obstruction on micturition reflex pathways in the rat. J Urol. 1988; 140:864–871.
55. Chai TC, Gray ML, Steers WD. The incidence of a positive ice water test in bladder outlet obstructed patients: evidence for bladder neural plasticity. J Urol. 1998; 160:34–38.
56. Hirayama A, Fujimoto K, Matsumoto Y, Ozono S, Hirao Y. Positive response to ice water test associated with high-grade bladder outlet obstruction in patients with benign prostatic hyperplasia. Urology. 2003; 62:909–913.
57. Hirayama A, Fujimoto K, Matsumoto Y, Hirao Y. Nocturia in men with lower urinary tract symptoms is associated with both nocturnal polyuria and detrusor overactivity with positive response to ice water test. Urology. 2005; 65:1064–1069.
58. Steers WD, Creedon DJ, Tuttle JB. Immunity to nerve growth factor prevents afferent plasticity following urinary bladder hypertrophy. J Urol. 1996; 155:379–385.
59. Speakman MJ, Brading AF, Gilpin CJ, Dixon JS, Gilpin SA, Gosling JA. Bladder outflow obstruction--a cause of denervation supersensitivity. J Urol. 1987; 138:1461–1466.
60. Boselli C, Govoni S, Condino AM, D'Agostino G. Bladder instability: a re-appraisal of classical experimental approaches and development of new therapeutic strategies. J Auton Pharmacol. 2001; 21:219–229.
61. O'Reilly BA, Kosaka AH, Chang TK, Ford AP, Popert R, McMahon SB. A quantitative analysis of purinoceptor expression in the bladders of patients with symptomatic outlet obstruction. BJU Int. 2001; 87:617–622.
62. Haferkamp A, Mundhenk J, Bastian PJ, Reitz A, Dorsam J, Pannek J, et al. Increased expression of connexin 43 in the overactive neurogenic detrusor. Eur Urol. 2004; 46:799–805.
63. Christ GJ, Day NS, Day M, Zhao W, Persson K, Pandita RK, et al. Increased connexin43-mediated intercellular communication in a rat model of bladder overactivity in vivo. Am J Physiol Regul Integr Comp Physiol. 2003; 284:R1241–R1248.
64. Kubota Y, Hashitani H, Shirasawa N, Kojima Y, Sasaki S, Mabuchi Y, et al. Altered distribution of interstitial cells in the guinea pig bladder following bladder outlet obstruction. Neurourol Urodyn. 2008; 27:330–340.
65. Kim SO, Oh BS, Chang IY, Song SH, Ahn K, Hwang EC, et al. Distribution of interstitial cells of Cajal and expression of nitric oxide synthase after experimental bladder outlet obstruction in a rat model of bladder overactivity. Neurourol Urodyn. 2011; 30:1639–1645.
66. Biers SM, Reynard JM, Doore T, Brading AF. The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int. 2006; 97:612–616.
67. Kubota Y, Biers SM, Kohri K, Brading AF. Effects of imatinib mesylate (Glivec) as a c-kit tyrosine kinase inhibitor in the guinea-pig urinary bladder. Neurourol Urodyn. 2006; 25:205–210.
68. Steers WD, Kolbeck S, Creedon D, Tuttle JB. Nerve growth factor in the urinary bladder of the adult regulates neuronal form and function. J Clin Invest. 1991; 88:1709–1715.
69. Liu HT, Kuo HC. Urinary nerve growth factor levels are increased in patients with bladder outlet obstruction with overactive bladder symptoms and reduced after successful medical treatment. Urology. 2008; 72:104–108.
70. Ha US, Park EY, Kim JC. Effect of botulinum toxin on expression of nerve growth factor and transient receptor potential vanilloid 1 in urothelium and detrusor muscle of rats with bladder outlet obstruction-induced detrusor overactivity. Urology. 2011; 78:721.e1–721.e6.
71. Yoshimura N, Seki S, Chancellor MB, de Groat WC, Ueda T. Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology. 2002; 59:5 Suppl 1. 61–67.
72. Yoshimura N, Birder L. Interstitial cystitis and related painful bladder syndromes: pathophysiology. In : Pasricha PJ, Willis WD, Gebhart GF, editors. Chronic abdominal and visceral pain: theory and practice. New York: Informa Healthcare USA;2007. p. 495–520.
73. Birder LA. Urothelial signaling. Handb Exp Pharmacol. 2011; (202):207–231.
74. Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999; 19:4644–4653.
75. Hayashi Y, Takimoto K, Chancellor MB, Erickson KA, Erickson VL, Kirimoto T, et al. Bladder hyperactivity and increased excitability of bladder afferent neurons associated with reduced expression of Kv1.4 alpha-subunit in rats with cystitis. Am J Physiol Regul Integr Comp Physiol. 2009; 296:R1661–R1670.
76. Lazzeri M, Beneforti P, Benaim G, Maggi CA, Lecci A, Turini D. Intravesical capsaicin for treatment of severe bladder pain: a randomized placebo controlled study. J Urol. 1996; 156:947–952.
77. Lazzeri M, Beneforti P, Spinelli M, Zanollo A, Barbagli G, Turini D. Intravesical resiniferatoxin for the treatment of hypersensitive disorder: a randomized placebo controlled study. J Urol. 2000; 164(3 Pt 1):676–679.
78. Payne CK, Mosbaugh PG, Forrest JB, Evans RJ, Whitmore KE, Antoci JP, et al. Intravesical resiniferatoxin for the treatment of interstitial cystitis: a randomized, double-blind, placebo controlled trial. J Urol. 2005; 173:1590–1594.
79. Mukerji G, Yiangou Y, Agarwal SK, Anand P. Transient receptor potential vanilloid receptor subtype 1 in painful bladder syndrome and its correlation with pain. J Urol. 2006; 176:797–801.
80. Dinis P, Charrua A, Avelino A, Yaqoob M, Bevan S, Nagy I, et al. Anandamide-evoked activation of vanilloid receptor 1 contributes to the development of bladder hyperreflexia and nociceptive transmission to spinal dorsal horn neurons in cystitis. J Neurosci. 2004; 24:11253–11263.
81. Charrua A, Cruz CD, Narayanan S, Gharat L, Gullapalli S, Cruz F, et al. GRC-6211, a new oral specific TRPV1 antagonist, decreases bladder overactivity and noxious bladder input in cystitis animal models. J Urol. 2009; 181:379–386.
82. Charrua A, Cruz CD, Cruz F, Avelino A. Transient receptor potential vanilloid subfamily 1 is essential for the generation of noxious bladder input and bladder overactivity in cystitis. J Urol. 2007; 177:1537–1541.
83. Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, et al. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol. 1999; 161:438–441.
84. Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol. 1997; 79:572–577.
85. Dmitrieva N, Shelton D, Rice AS, McMahon SB. The role of nerve growth factor in a model of visceral inflammation. Neuroscience. 1997; 78:449–459.
86. Chuang YC, Fraser MO, Yu Y, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol. 2001; 165:975–979.
87. Evans RJ, Moldwin RM, Cossons N, Darekar A, Mills IW, Scholfield D. Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol. 2011; 185:1716–1721.
88. Kashyap M, Kawamorita N, Tyagi V, Sugino Y, Chancellor M, Yoshimura N, et al. Down-regulation of nerve growth factor expression in the bladder by antisense oligonucleotides as new treatment for overactive bladder. J Urol. 2013; 190:757–764.
89. Kebapci N, Yenilmez A, Efe B, Entok E, Demirustu C. Bladder dysfunction in type 2 diabetic patients. Neurourol Urodyn. 2007; 26:814–819.
90. Lee WC, Wu HP, Tai TY, Yu HJ, Chiang PH. Investigation of urodynamic characteristics and bladder sensory function in the early stages of diabetic bladder dysfunction in women with type 2 diabetes. J Urol. 2009; 181:198–203.
91. Ueda T, Yoshimura N, Yoshida O. Diabetic cystopathy: relationship to autonomic neuropathy detected by sympathetic skin response. J Urol. 1997; 157:580–584.
92. Yoshimura N, Chancellor MB, Andersson KE, Christ GJ. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int. 2005; 95:733–738.
93. Kaplan SA, Te AE, Blaivas JG. Urodynamic findings in patients with diabetic cystopathy. J Urol. 1995; 153:342–344.
94. Daneshgari F, Liu G, Birder L, Hanna-Mitchell AT, Chacko S. Diabetic bladder dysfunction: current translational knowledge. J Urol. 2009; 182:6 Suppl. S18–S26.
95. Torimoto K, Hirao Y, Matsuyoshi H, de Groat WC, Chancellor MB, Yoshimura N. alpha1-Adrenergic mechanism in diabetic urethral dysfunction in rats. J Urol. 2005; 173:1027–1032.
96. Torimoto K, Fraser MO, Hirao Y, De Groat WC, Chancellor MB, Yoshimura N. Urethral dysfunction in diabetic rats. J Urol. 2004; 171:1959–1964.
97. Steinbacher BC Jr, Nadelhaft I. Increased levels of nerve growth factor in the urinary bladder and hypertrophy of dorsal root ganglion neurons in the diabetic rat. Brain Res. 1998; 782(1-2):255–260.
98. Sasaki K, Chancellor MB, Phelan MW, Yokoyama T, Fraser MO, Seki S, et al. Diabetic cystopathy correlates with a long-term decrease in nerve growth factor levels in the bladder and lumbosacral dorsal root Ganglia. J Urol. 2002; 168:1259–1264.
99. Anand P, Terenghi G, Warner G, Kopelman P, Williams-Chestnut RE, Sinicropi DV. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat Med. 1996; 2:703–707.
100. Tomlinson DR, Fernyhough P, Diemel LT. Role of neurotrophins in diabetic neuropathy and treatment with nerve growth factors. Diabetes. 1997; 46:Suppl 2. S43–S49.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download