INTRODUCTION
Testicular tumors are rare in children compared with those occurring postpubertally or with other genitourinary tumors in childhood such as Wilms' tumor. A few large-scale reports have been published regarding pediatric testicular tumors [1,2,3,4,5]. In 1980, the Urologic Section of the American Academy of Pediatrics (AAP) began a Prepubertal Testicular Tumor Registry. The AAP released their data on the demographics and natural history of prepubertal testicular tumors in 1988, 1993, and 2002, respectively [1,2,3]. To our knowledge, there have been a few reports on pediatric testicular tumors from a single institution in Korea [6]. There were two large studies for registry of pediatric testicular tumors in 2004 and 2011 in Korea [7,8].
Pediatric testicular tumors are distinct from adult testicular tumors. Germ cell tumors in adults represent about 95% of all testicular tumors. In children, however, germ cell tumors represent only 60% to 75% of testicular tumors. Whereas only a small number of adult testicular tumors are benign, one-quarter to one-third of pediatric testicular tumors are benign [9]. Collectively, these data on pediatric testicular tumors suggest that prepubertal tumors should be managed in a manner different from that for adult testicular tumors.
A testis-sparing approach is more common owing to the high incidence of benign tumors in children. Orchiectomy and observation with very selective chemotherapy has become the standard approach when a malignant tumor is identified. Retroperitoneal lymph node dissection (RPLND) and radiation therapy play very limited roles [10].
In this review, we summarize the contemporary literature on pediatric testicular tumors, with a focus on their management.
INCIDENCE
Pediatric testicular tumors, which account for 1% to 2% of all pediatric solid tumors, are less frequent in childhood than at postpubertal ages. The annual incidence is 1.6 (0.5 to 2) per 100,000 for boys less than 15 years of age [11,12]. The incidence of prepubertal testicular tumors in Korea was reported to be 0.98 per 100,000 children, which is close to the incidence reported in other countries [9]. Pediatric testicular tumors may occur in all age groups with the following two peaks: one peak before 3 years of age and one peak at the postpubertal period [13]. It was reported that the incidence of childhood testicular tumors peaks at 2 years of age, tapers off after 4 years of age, and then begins to rise again at puberty [14]. This bimodal age distribution indicates fundamental differences between prepubertal and postpubertal testicular tumors. Moreover, prepubertal and postpubertal testicular tumors show many other differences including the typical tumor histology, molecular biological differences, and the malignant potential of tumors at different ages [10].
According to race, testicular tumors during infancy are more common in whites and are rarer in black and Asian children [11]. In Korea, two large-scale studies registered pediatric testicular tumors in 2004 and 2011, respectively [7,8]. The incidence rate of pediatric testicular tumors greatly decreases after 4 years of age. Compared with the data obtained from the United States (76% of tumors before 2 years of age and 20% between 2 and 4 years of age) [2], the incidence rate of pediatric testicular tumors before 4 years of age in Korea is slightly lower (Fig. 1). In infants and children older than 5 years of age, benign tumors are more common than yolk sac tumors. Pediatric testicular tumors occurred at all ages in both studies.
ETIOLOGY
Although etiologic factors have been studied in a number of reports in the literature, the definite etiology of testicular cancer has remained unclear until now. Patients with disorders of sex development have been reported to have an increased incidence of gonadal tumors. Patients with hypovirilization and gonadal dysgenesis are exposed to the highest risk. The risk of tumor formation in gonadal dysgenesis is increased if there is a Y chromosome present. The incidence of tumor development was reported to increase approximately 10% by 20 years of age [14]. Intratubular germ cell neoplasia (ITGCN) has been reported in 6% of children with disorders of sex development. However, it has a higher incidence rate after puberty [15]. The association between cryptorchidism and germ cell tumors of the testis is well known [2]. Germ cell tumors are most often associated with bilateral cryptorchidism [16]. The relative risk of cancer increases with age at orchiopexy, with the greatest risk after puberty [17].
PATHOLOGY
Testicular tumors are generally classified by their putative origin. Pediatric testicular tumors include germ cell and gonadal stromal (nongerm cell) tumors. Germ cell tumors include yolk sac tumors, teratoma, epidermoid cysts, seminoma, choriocarcinoma, and embryonal carcinoma. Epidermoid cysts are generally considered as a monodermal form of teratoma. Gonadal stromal (nongerm cell) tumors include Leydig cell tumor, Sertoli cell tumor, juvenile granulosa cell tumor, and gonadoblastoma. Testicular tumors may also be classified as either benign or malignant on the basis of their clinical behavior. Yolk sac tumors, seminoma, choriocarcinoma, and embryonal carcinoma are malignant. Teratomas are universally benign in prepubertal children but may be malignant in adolescents and adults. Benign teratoma is the most common germ cell tumor in children. The most common malignant tumor in children is yolk sac tumor, which is very rare in its pure form in postpubertal patients. Most gonadal stromal tumors are benign, although they can occasionally be malignant, especially in older children [10].
Yolk sac tumor and teratoma are common according to two large-scale Korean studies conducted for the registry of pediatric testicular tumors [7,8]. From 1997 to 2001, germ cell tumors were the most common (88.7%), mainly yolk sac tumor (41.8%) or teratoma (35.7%). Potentially malignant tumors accounted for 52.5% of patients, and the remaining tumors were generally benign. From 2002 to 2008, germ cell tumors were the most common (79.1%), including yolk sac tumor (29.4%) and teratoma (39.2%).
A greater percentage of benign lesions occur in children than in adults. Overall, approximately 75% of testicular tumors in prepubertal children are benign [12]. In contrast, the large majority of testicular tumors in adolescents and adults are malignant germ cell tumors [10]. Although approximately 75% of prepubertal testicular tumors were reported to be malignant in one report [18], benign lesions were found to be more frequent in pediatric testicular tumors in many recent reports. For example, a recent multicenter report found that 74% of primary testicular tumors in prepubertal children were benign [12].
Pediatric testicular tumors have distinct molecular biological and histological characteristics. For example, chromosome 12 abnormalities are seen in nearly all adult malignant germ cell tumors, but are not seen in prepubertal yolk sac tumors, which display abnormalities in other chromosomes [19]. ITGCN, which is frequently seen in the testicles of men with malignant germ cell tumors, is absent in prepubertal yolk sac tumors [20]. Moreover, ITGCN is generally absent in prepubertal testicles harboring a teratoma, whereas 88% of testes removed from adult teratoma contain areas of ITGCN [10,21,22,23].
DIAGNOSIS AND STAGING
Physical examination is very important for the diagnosis of pediatric testicular tumors. The majority of pediatric testicular tumors present as a palpable testicular mass detected by the patient, by a parent, or by a physician on a routine physical examination. The testicular mass presents as a hardened, painless mass associated with an increase in scrotal volume. Disorders that must be excluded from pediatric testicular tumors are as follows: epididymitis, hydrocele, hernia, and spermatic cord torsion.
Serum tumor markers play critical roles in the diagnosis and surveillance of testicular tumors. Testicular tumor markers such as human chorionic gonadotropin (hCG) and alpha-fetoprotein (AFP) are important tools for the evaluation of testicular tumors in children and adolescents. Because yolk sac tumors do not increase the hCG level, AFP is the most important tumor marker in prepubertal patients. Blood levels of AFP are increased in most cases of malignant prepuberal testicular tumors [14]. AFP is elaborated by 90% of yolk sac tumors in children. It is important to know that AFP levels may be elevated in infant boys. Therefore, normal adult reference laboratory values for AFP should not be used for young children because AFP synthesis continues after birth [14,24,25,26]. Normal adult levels (<10 mg/mL) are not reached until 8 months of age [24]. However, hCG blood levels are rarely increased in pediatric testicular tumors.
In AAP reports, the overall AFP level was increased in 77.1% of patients and in 92% of yolk sac tumors. However, the β-hCG level was normal in all patients [2,11]. In a large-scale Korean study in 2004, in prepubertal testicular tumors, the most common symptom was a palpable scrotal mass in 79.9% of cases [9]. Other complaints included scrotal swelling (7.2% of cases), hydrocele (5.3%), scrotal pain (1.4%), trauma (0.5%), and others (5.7%). Of the total tumors, 51.7% were detected in the right testis, 46.9% were detected in the left testis, and 1.4% were bilateral, which suggests no predilection for laterality. Serum tumor marker assays revealed that serum AFP levels increased preoperatively in 62.9% of patients, particularly in 94.7% of patients with yolk sac tumors. AFP levels also increased in 30.4% of patients with teratoma. Preoperative β-hCG levels were elevated in 2.7% of patients (2.2% with yolk sac tumor and 2.7% with teratoma). Most of the preoperative diagnoses (66.5%) were malignant tumor, followed by benign tumor (22.0%), hydrocele (6.2%), and others (5.3%).
Occasionally, some patients present with hydroceles. Therefore, if a child has a hydrocele on the physical examination, testicular ultrasonography should be performed to evaluate the testicular mass. The ultrasonographic appearance of specific testicular tumors can be identified. For example, benign tumors tend to be well circumscribed with sharp borders and decreased blood flows on Doppler studies.
Yolk sac tumors tend to be more solid in appearance, and epidermoid cysts usually demonstrate echogenic debris within a well-defined cyst. In addition, ultrasonography distinguishes testicular masses from extratesticular masses [5,10]. Color Doppler ultrasonography is reported to be more effective than gray-scale ultrasonography for the detection of intratesticular neoplasms in pediatric patients [27]. To date, however, there are no reliable sonographic findings to distinguish benign tumors from malignant tumors. Nevertheless, the finding of anechoic cystic lesions may suggest a benign lesion, such as an epidermoid cyst or teratoma. Therefore, ultrasonographic features can be used for the suggestion of the tumor, but not for its diagnosis.
Radiographic evaluation of children with a suspected testicular tumor is very limited. Since many prepubertal testicular tumors are benign, any metastatic evaluation is deferred until tissue confirmation of a malignancy. When both clinical findings and ultrasonography images suggest a benign tumor, no further evaluation is necessary. In cases in which malignancy is suspected, it is necessary to search for retroperitoneal and pulmonary metastases with a computed tomography (CT) scan of the abdomen and the pelvis. A CT scan of the retroperitoneum can identify most patients who have lymph node metastases. However, there is a 15% to 20% false-negative rate [28,29]. A chest x-ray or chest CT can identify pulmonary metastases.
Currently, there is no universally accepted staging system for pediatric testicular tumors. Prepubertal patients are generally staged on the basis of the extent of the locally confined disease, the presence or absence of metastatic disease based on radiographic imaging, and the persistence or decline of tumor marker levels postoperatively. According to the Children's Oncology Group staging system, patients with pediatric testicular tumors are designated as Stages 1 to 4. However, adolescents with germ cell tumors are generally staged as adults on the basis of the TNM system recommended by the American Joint Committee on Cancer and the International Union Against Cancer [10,30].
TREATMENT
Consensus is lacking on the most appropriate therapeutic approach for different stages of pediatric testicular tumors because limited numbers of large-scale studies have been reported in the literature [13]. Whereas inguinal radical orchiectomy with an early ligation of the cord has been the historical approach for testicular tumors, consideration of testis-sparing surgery for benign testicular tumors has increased [3]. Surgical treatment usually begins with radical orchiectomy, which is often recommended whenever the AFP level is elevated. An elevated AFP level in a child over 1 year of age often suggests the presence of a yolk sac tumor. A normal AFP level in children suggests a strong likelihood of a benign tumor. For such tumors, as in cases of teratoma and epidermoid cyst, testis-sparing surgery of the mass rather than radical orchiectomy should be taken into consideration. This is also true in boys presenting with androgenization.
In adolescents and adults, a testis-sparing approach must be used judiciously because most postpubertal tumors are malignant. However, in adolescents with normal tumor marker levels and ultrasonography images highly suggestive of benign lesions such as epidermoid cysts, a testis-sparing approach may be considered [10].
Following the excision of the primary tumor, benign tumors usually do not require further evaluation or treatment. Treatments for potentially malignant tumors include surveillance, chemotherapy, RPLND, and radiation therapy. In the case of malignant stage I testes tumors, surgery alone yields excellent survival rates, with recurrence in 15.5% of the patients. Stage II disease has a recurrence rate of 75%. In both cases, recurrence can be successfully treated with platinum-based multiagent chemotherapy. Higher stages of the disease also have excellent prognosis using both surgery and chemotherapy [31].
Whether RPLND is necessary for pediatric testicular tumors is controversial. RPLND plays an important staging and therapeutic role for mixed germ cell tumors in adolescents. However, it is rarely used in prepubertal yolk sac tumors. Unlike adult testicular tumors, hematogenous spread is more common in children [2,18,32,33,34,36,37]. The incidence of metastasis in children with stage I disease is 16%. Of these, 24% are expected to have disease solely in the retroperitoneum [37]. However, RPLND after radical orchiectomy seems to be unnecessary in pediatric patients, especially those younger than 10 years of age with radiologic evidence of negative nodal involvement [13]. RPLND is not beneficial either as a staging procedure or as a therapeutic procedure in stage I, group I, or group IIa diseases of these tumors [13]. RPLND should be reserved for the following: (1) malignant germ cell tumor patients who have persistently elevated AFP levels after orchiectomy with a normal total-body CT scan, and for patients presenting with stage II or stage III disease with definitive abnormality on CT scans, and (2) group IIb, IIc, and stage III paratesticular rhabdomyosarcoma patients with radiologic evidence of retroperitoneal involvement on CT scans.
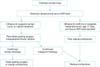
In a large-scale Korean study on prepubertal testicular tumors in 2004, the majority of patients (96.6%) were treated with radical orchiectomy, whereas 2.9% and 0.5% of patients underwent simple and partial orchiectomy, respectively. Of all patients, 71.8% were on surveillance only after surgery. Subsequently, 1.9% of this group received additional therapy, including chemotherapy and/or RPLND and/or radiation therapy owing to metastasis. After surgery, 9.1% and 1.4% of patients received chemotherapy alone or combination therapy (chemotherapy, RPLND, and radiotherapy), respectively. All patients with stage I yolk sac tumors were on surveillance only after surgery. Of these patients, 5.9% subsequently had metastasis. On the basis of these findings from Korea and other collaborative data reported in the literature, we suggest an algorithm for the management of prepubertal testicular tumors, which was suggested previously by the Korean Society of Pediatric Urology (Fig. 2) [9].
GERM CELL TUMORS
1. Yolk sac tumor
Yolk sac tumor is a common prepubertal testicular tumor of germ cell origin [4,38]. This tumor occurs primarily in children younger than 2 years of age. It presents as a solid testicular mass associated with increased levels of AFP [11]. Most cases present as localized diseases, and only 4% to 6% patients have retroperitoneal or pulmonary metastases [39,40]. More than 90% of prepubertal children present with stage I disease [41]. Nearly all yolk sac tumors are managed with surveillance or platinum-based chemotherapy. RPLND is rarely used in children with yolk sac tumors.
The initial treatment for a yolk sac tumor is radical inguinal orchiectomy. This treatment is curative for yolk sac tumors in most children. Routine RPLND or adjuvant chemotherapy is not recommended [41,42,43]. Clinical stage I patients do not need to receive additional adjuvant treatment after radical orchiectomy. Follow-up monitoring with chest x-ray, CT, or magnetic resonance imaging of the retroperitoneum is recommended until the patient is 36 months posttreatment. Moreover, measurement of tumor marker levels and a physical examination need to be performed at a more frequent interval. Patients who have undergone prior scrotal biopsy are considered as stage II [44]. A completion orchiectomy after removing all cord structures and an abdominal CT scan to examine for retroperitoneal lymphadenopathy are needed.
Patients who have persistent elevation of the AFP level and retroperitoneal adenopathy are likely to have metastatic disease. These patients can be treated as stage III. Cases with a persistently elevated AFP level or confirmed metastases must be treated with chemotherapy, which has a 6-year survival rate of nearly 100% [42,44,45]. Combination chemotherapy using platinum-based therapy (cisplatin, etoposide, and bleomycin) has been used in pediatric patients with advanced germ cell tumors [46].
2. Mature teratoma
Teratoma accounts for 30% to 40% of testicular tumors in prepubertal children, with a peak incidence at 13 months of age [4,12,38,42]. Prepubertal mature teratomas have a benign clinical course that is different from the clinical behavior of teratomas in adults. For these tumors, no oncological evaluation or follow-up is necessary [47,48]. Although not specific, a presumptive diagnosis of teratoma can often be made by ultrasonography examination. Testicular teratomas are currently managed with testis-sparing procedures rather than radical orchiectomy [4,5,21,42,49]. Recurrence of testicular teratomas rarely occurs. Frozen sections obtained intraoperatively can reliably distinguish a benign tumor from malignant lesions [5,50]. However, adolescents with teratoma should undergo orchiectomy like adults and a metastatic evaluation should be followed by surveillance for stage 1 disease [10].
3. Immature teratomas
Immature teratomas have often been considered to be malignant tumors. They appear to be benign in children unless they have foci of malignant cells. Most patients with recurrent tumors can be salvaged with platinum-based chemotherapy; therefore, testicular immature teratomas have a low risk for relapse [14,48]. Observation alone is recommended for completely resected immature teratomas, although there has been at least one case report of metastatic disease resulting from an immature testicular teratoma in a child [42,48,51,52]. Although immature teratomas of the testis can be managed with complete tumor resection alone, clinical and radiographic follow-up should be considered for these patients [10].
4. Epidermoid cyst
Epidermoid cysts probably represent a monodermal variant of teratoma. Epidermoid cysts account for 15% of pediatric testicular tumors [5,12]. Since epidermoid cysts are usually benign, testis-sparing surgery has been advocated in both children and adults [12,22,53]. The diagnosis of epidermoid cysts can be suggested by ultrasonography; the cysts are easily identified owing to their peculiar aspect of concentric layers similar to those of an onion [54]. Frozen section diagnosis has been shown to be a reliable method for distinguishing this lesion [5,50].
GONADAL STROMAL TUMORS
Stromal tumors of the testis are rare in children and adolescents. Leydig cell tumors and juvenile granulosa cell tumors are universally benign in children [10,55,56,57]. Although approximately 10% of Sertoli cell tumors are malignant in adults, malignancy is rare in children [55,58].
1. Leydig cell tumors
These are the most common of the sex cord tumors and show a peak incidence at 4 to 5 years of age. Because Leydig cells produce testosterone, the production of testosterone by the tumor cells can result in precocious puberty [59]. Inguinal radical orchiectomy has been most often used for treatment of Leydig cell tumors. Testis-sparing surgery and enucleation of the tumor has now been used for a number of children [5,60,61]. Local recurrence was reported in one patient [62]. Malignancy of Leydig cell tumors has not been reported in children [40].
2. Juvenile granulosa cell tumors
Juvenile granulosa cell tumors occur almost only in the first year of life, most in the first 6 months. They show a light microscopic appearance similar to that of ovarian juvenile granulosa cell tumors. Chromosomal mosaicism, structural abnormalities of the Y chromosome, and ambiguous genitalia are commonly found in boys with juvenile granulosa cell tumors [10,63,64]. Therefore, these children should undergo a chromosomal analysis. The tumor itself may be treated by orchiectomy or tumor enucleation without metastatic evaluation or adjuvant therapy [10,57,65].
3. Sertoli cell tumor
Sertoli cell tumor is the next most common pediatric gonadal stromal tumor following Leydig cell tumor [58]. It occurs from 4 months of age to 10 years of age with a usual presentation of a painless testicular mass. Although these tumors are hormonally inactive, they can occasionally cause gynecomasty or early puberty [66]. There are limited series of pediatric patients with Sertoli cell tumors [55,66,67]. Observation is mostly recommended because metastases are rare [68]. The vast majority of patients have been managed with orchiectomy. Testis-sparing procedures have also been performed in prepubertal children [5,36,69]. Although all reported cases of Sertoli cell tumors in children under 5 years of age have been benign, a few cases of malignant Sertoli cell tumors in older children have been reported [63,68]. Examination of the retroperitoneum is needed to exclude retroperitoneal spread in older boys [70].
4. Gonadoblastoma
Gonadoblastomas are the most common tumors found to have an association with sex differentiation disorders. Gonadoblastomas are usually benign and asymptomatic, but sometimes may be associated with virilization. Gonadoblastomas occur in dysgenetic gonads in association with the presence of the Y chromosome in the karyotype [71]. Children with mixed gonadal dysgenesis have a 25% risk of malignancy formation [72], and the incidence rate increases with age [71]. Therefore, all streak gonads and undescended testes should be removed. Early gonadectomy is advocated because tumors have been reported in children under 5 years of age [73,74]. When malignancies occur, dysgerminoma is the most common histological type. These tumors are very radiosensitive. Therefore, the outlook for these patients is generally favorable [75].
OTHER LESIONS
Leukemia and lymphoma are the most common malignancies that spread to the testicles in children among other lesions. Children with acute lymphoblastic leukemia who have bulky diseases at diagnosis have been reported to have up to a 20% incidence of testicular relapse [76]. Follicular lymphoma may occur as the primary tumor of the testis [77]. The localized testicular tumor has favorable prognosis. Testicular involvement occurs in 4% of boys with Burkitt lymphoma, which might be the initial clinical presentation [78].
Testicular cystic dysplasia is a rare benign lesion in children with an increasing frequency [79,80]. In this case, management options are testis-sparing surgery or nonoperative treatment with serial testicular ultrasonography [79,81].
More than 5% of healthy young men present with testicular microlithiasis. Testicular microlithiasis has been reported to be associated with testicular tumors [14]. There are still insufficient data to quantify the risk of testicular cancer in boys or men incidentally found to have testicular microlithiasis. Testicular microlithiasis has been noted less often in children. Noninvasive ultrasonography follow-up until adult age is recommended for testicular microlithiasis [82,83,84].
CONCLUSIONS
The incidence of pediatric testicular tumors is usually very low. Most pediatric testicular tumors occur before 3 years of age or during the postpubertal period. Pediatric testicular tumors are very different from those in adults in terms of benign or malignant status and cell types. Thus, the management of these pediatric patients should also differ from that of adults. Malignant pediatric testicular tumors are mostly treatable and potentially curable. However, owing to the relatively low prevalence of abnormal serum tumor markers and metastasis, meticulous and careful follow-up is required for children. In addition, attention should be given to options for testis-sparing surgery in benign tumors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download