Abstract
In terms of treating diseases, minimally invasive treatment has become a key element in reducing perioperative complications. Among the various minimally invasive treatments, cryotherapy is often used in urology to treat various types of cancers, especially prostate cancer and renal cancer. In prostate cancer, the increased incidence of low-risk, localized prostate cancer has made minimally invasive treatment modalities an attractive option. Focal cryotherapy for localized unilateral disease offers the added benefit of minimal morbidities. In renal cancer, owing to the increasing utilization of cross-sectional imaging, nearly 70% of newly detected renal masses are stage T1a, making them more susceptible to minimally invasive nephron-sparing therapies including laparoscopic and robotic partial nephrectomy and ablative therapies. This article reviews the various outcomes of cryotherapy compared with other treatments and the possible uses of cryotherapy in surgery.
Low temperature as a treatment method has been used in medicine for a long time for its anesthetic effect. Cryotherapy, or tissue destruction by deep freezing and thawing, is well known for its use in treating and managing both benign tumors and localized cancer [1]. Although cold temperature has been used therapeutically for centuries, Arnott [2] was the first to use low temperature to treat cancer in the middle of the 19th century when he used iced saline solutions to manage advanced breast and uterine cancers. By the end of the 19th century and beginning of the 20th century, liquid air and liquid oxygen came into use for treating various skin diseases [3]. Cryotherapy applications began to grow rapidly in 1961, when Cooper and Lee [4,5] came up with a cryosurgical unit capable of delivering liquid nitrogen (-196℃), which they used for treating Parkinsonism.
In addition to Cooper's use of cryotherapy in treating Parkinsonism, the modified version by Gonder et al. [6,7] initiated the use of cryotherapy in urology by using the Cooper's unit for prostate ablation. Their procedure involved transurethral freezing with a single closed-probe system using liquid nitrogen.
Although cryosurgery came into use for treating patients unsuitable for surgery, local complications were common and as better treatment modalities became available for benign prostatic hypertrophy, cryosurgery in treating urologic conditions was abandoned.
Nevertheless, cryosurgery continued to develop in other specialties, with improvements made in liquid nitrogen probes and heating devices [8,9,10]. In the 1980s, Korpan [11] performed experiments to understand the mechanism of cell damage caused by freezing and along with his clinical studies, the experiment provided the framework for the formulation of clinical and technical requirements for modern cryosurgery.
As the demand for minimally invasive techniques increased and as more effective cryosurgical units were developed, cryosurgery reemerged about a decade ago as a clinical method in the field of urologic oncology. Furthermore, with the development of the percutaneous transperineal approach by Onik et al. [12], the use of cryotherapy in treating localized prostate cancer started to receive the spotlight. Since then, cryotherapy has been widely used in treating both benign and malignant prostatic diseases [13].
Cryotherapy stimulates tumor cell death in the following two ways: directly by damaging cell membranes and organelles and indirectly by initiating vascular compromise through thrombosis of small vessels [14,15]. With lowering of temperatures, the cells dehydrate and proteins become damaged owing to the high solute concentration, resulting in disruption of the membranes and malfunctioning of the enzymatic machinery of the cell. Ice crystals form inside the cells with faster cooling, which leads to mechanical damage to cell membranes and organelles. As the process is repeated, the thermal conductivity of the tissue increases and the damage is spread.
Vessel wall damage can occur as the result of perivascular cellular hydration, resulting in vessel distension and mechanical injury, or from direct cell damage to endothelial cells lining the vessels. Both pathways eventually lead to increased permeability, edema, and a coagulation cascade, leading to microthrombi in vessels and tissue ischemia. Reperfusion injury is also known to play a role in the cell damage.
In histology, the mechanisms mentioned earlier result in coagulative necrosis of the tissue. According to Sindelar et al. [16], who studied the effects of cryosurgery in rat kidney, cellular proteins are denatured and membranes are disrupted within 1 hour of cryosurgery. In 24 hours after cryosurgery, the tissue undergoes complete coagulative necrosis, along with mild to moderate infiltration of lymphocytes, plasma cells, and macrophages. Therefore, freezing of tissue results in the formation of inflammatory debris.
The use of cryotherapy has increased considerably and it was recognized as a therapeutic option for prostate cancer by the American Urological Association (AUA) in 1996. With vast technological improvements in third-generation systems, the procedure, with its minimal morbidity, became acceptable therapy for both primary and salvage treatment of prostate cancer [17]. The process of targeted cryosurgical ablation of the prostate (T-CSAP) is shown in Fig. 1.
A systematic review of peer-reviewed publications concluded that the level of evidence for effectiveness of primary cryosurgery for clinically localized prostate cancer was low [18]. However, these data showed a combination of older and newer cryo systems. Although not directly comparable owing to the nature of the study designs and definition of recurrence, cryotherapy seems to have similar short-term results with respect to biochemical control compared with other methods [13].
Chin et al. [19] reported the results of a randomized trial comparing radiation therapy with therapeutic effect in T2c and T3b prostate cancer. Although they were planning on comparing 150 patients, 21 of 33 patients who received cryotherapy and 14 of 31 patients who received radiation therapy ended up as failures by the American Society for Therapeutic Radiation Oncology (ASTRO) definition. Hence, only 64 patients were enrolled in the study. Even though the disease-specific survival rate and overall survival rate were similar in the two groups, the average disease-free survival rate was higher in the group treated by radiation. According to this report, seven patients from the cryotherapy group and four patients from the radiation therapy group showed positive results in the follow-up biopsy.
Presently, either the ASTRO or the Phoenix criteria are used as the primary method for detecting recurrence after cryosurgery. Nonetheless, these criteria were originally designed for assessing radiation therapy outcomes and hence could cause confusion in the results. Several studies define recurrence on the basis of prostate-specific antigen (PSA) levels with cutoff values of less than 0.5 ng/mL. Overall, these differences in criteria led to limitations in comparing and analyzing the efficacy of cryosurgery with alternative therapeutic interventions [20].
Despite the limitations, long-term data for prostate cryosurgery have been reported; a retrospective review by Cohen et al. [21] reported 10-year biochemical disease-free survival (bDFS) of 56.01% according to the ASTRO criteria and 62.36% according to the Phoenix criteria. Furthermore, when categorizing these patients on the basis of risk groups (low, medium, and high), bDFS using the Phoenix criteria was 80.56%, 74.16%, and 45.54%, respectively. The overall 10-year survival rate for negative biopsy status was 73.81%. Recently, a large study cohort, the Cryo On-Line Database (COLD) Registry, was designed to address the limited amount of data on cryotherapy and integrated data from 4 academic medical centers and 34 community urologists [17]. Five-year data from the COLD Registry reported bDFS of 77.1% by use of the ASTRO criteria and 72.9% by use of the Phoenix criteria for the entire cohort. After risk stratification, the 5-year bDFS was 84.7%, 73.4%, and 75.3% (ASTRO) and 91.1%, 78.5%, and 62.2% (Phoenix) for low-, medium-, and high-risk groups, respectively. Although the reported long-term data are interesting, because there has been a shift in technology from second- to third-generation systems, the outcomes may not necessarily reveal the efficacy of cryosurgery [13].
With the use of third-generation technology, complications occurring secondary to cryosurgery have been reduced dramatically. According to Han et al. [22], in a multicenter series of 106 patients, the rate of complications reported was only 5% for urethral sloughing, 3% for incontinence requiring pads, 5% for urge incontinence requiring no pads, 3.3% for transient urinary retention, and 2.6% for rectal pain. Additionally, none of these patients developed recto-urethral fistulas. Even lower rates of urethral sloughing (2%) and incontinence (2%) have been reported in single-institution experiences with third-generation systems [23]. Hubosky et al. [23] reported that with 18-month follow-up, cryosurgery patients had better urinary function compared with a series of brachytherapy patients, and this improvement was still present at 24 months. However, sexual dysfunction in this group of cryosurgery patients remained problematic as the entire cohort only achieved a 20% return to baseline sexual function at the 12-month follow-up.
Even though 83% of patients had an absence of biochemical recurrence after radiotherapy, when followed for 5 years, the positive posttreatment biopsy rate for prostate cancer varied from 21% to 51% [24,25]. Salvage prostatectomy for local disease recurrence after radiation therapy is technically challenging and is associated with increased morbidity from rectal injury (2%), urinary incontinence (23%), and anastomotic stricture (30%) [26]. Cryosurgery provides an effective substitute for salvage therapy with a less invasive approach and lower complication rates than for radical surgery, with an incontinence rate of 3% and a urethra-rectal fistula rate of 2% [27]. The 5-year overall survival rate for salvage cryotherapy is approximately 97% and the biochemical failure rate is reported to be 44% to 59% [27,28]. In the largest combined series with 279 patients, analysis of the COLD Registry reported a 59% 5-year bDFS rate by use of the ASTRO criteria and a 54.5% rate by use of the Phoenix criteria [28]. In other series, using risk stratification, Stephenson et al. [26] reported 5-year bDFS rates of 73%, 45%, and 11% for low-, medium-, and high-risk groups, respectively. These modest salvage therapy outcomes were further corroborated by Izawa et al. [27], who reported a positive post-salvage-biopsy rate of 23%, and by Chin et al. [29], who observed an even lower rate (14.2%).
Generally, it is known that all prostate therapies can leave viable cells. Because complete ablation of prostatic tissue is challenging, particularly in the periurethral region where the urethral warmer protects the tissue from reaching the necessary lethal low temperatures, viable tissue and tumor can be found on posttreatment prostate biopsies. Izawa et al. [30] showed that among 158 men undergoing post salvage prostate biopsy or transurethral prostate resection, 27 (17%) were found to have residual cancer.
Patients (8.2%) in the COLD Registry received posttreatment hormone ablation, and Ng et al. [31] reported an even higher percentage of patients using ADT (32%). ADT is used to reduce the prostate gland and minimize the possibility of unsuccessful freezing and ablation; nonetheless, most patients treated in the salvage setting have relatively small glands [32].
Salvage cryotherapy seems to be well endured with a urinary incontinence rate of 4.7% and a fistula rate of 1.2% stated in the COLD Registry [28]. Furthermore, a single-institution series reported a higher incontinence rate of 13% but a similar recto-urethral fistula rate of 1% [33].
With the improvements in cryo-technology, especially the use of the thermocouple monitor in the area of the sphincter, the incidence of urinary complications has decreased. Whereas the incontinence rate was as high as 83% in earlier experiences with older cryo-technology, more recent studies using third-generation technology have demonstrated a substantial decrease in the incontinence rate (9%) [34,35]. However, the rates of impotence after salvage cryotherapy are still high owing to the inevitable damage to the neurovascular bundle, with up to 90% of patients being affected [36]. The current ability to maintain the temperature along multiple points in the bundle may decrease erectile dysfunction in the future.
Although radical prostatectomy has been widely used as the standard of care for a long time, the method has substantial morbidity. On the other hand, cryosurgical treatment has had extensive trials and has been recognized as an acceptable alternative to radical excision [21,37]. However, cryosurgery too has morbidity related to freezing extra prostatic tissues during ablation of the prostate. This, in turn, has led to recent high interest in focal treatment by cryosurgery, a technique of unilateral nerve-sparing ablation introduced by Onik et al. [38]. Recently, a variety of ablative techniques including magnetic resonance imaging (MRI)-guided laser ablation and MRI-guided focused ultrasound, which are considered to be promising options in therapy, are used in focal treatment [39,40,41]. Only a few percentages of prostate cancers, however, are sufficiently localized to be considered for focal therapy. Thus, this method is viewed as a treatment choice between radical treatment methods, such as prostatectomy and radiotherapy, and active surveillance. The major challenge to focal treatment effectiveness relates to the ability to anatomically localize portions of the gland containing cancer versus those that are cancer-free. Clinical trials focused on evaluating focal therapy are needed but will be complex and difficult.
Onik et al. [42] were the first to report the use cryotherapy as a focal therapy; they reported on nine patients with an average follow-up of 3 years. All of the patients had a stable PSA at the time of reporting, and in addition, seven of the nine previously potent men maintained erectile function adequate for penetration.
Among three patients who underwent bilateral gland ablation, one patient had the tumor ablated with a margin around the tumor and the other two patients received hemiablation. A follow-up report included 48 patients with a follow-up of at least 2 years and an average follow-up of 4.5 years [38]. Forty-five of 48 men had a stable PSA by the ASTRO definition and potency was preserved in 36 of 40 men (90%).
The COLD Registry is known to contain one of the largest data sets for patients treated with cryotherapy [43]. In the study, a total of 1,160 patients were treated with focal therapy and the authors mentioned that focal cryosurgery represented only 2.1% of the treatments used for patients entered into the database in 1999. However, by 2007, the percentage had increased to 38.2%. Of the patients treated with focal cryotherapy, 47%, 41%, and 12% were categorized into low-, intermediate-, and high-risk groups, respectively. The 3-year biochemical-free survival was 74.7%, which fairly is comparable to patients in the COLD Registry treated with whole gland cryotherapy [43]. Preservation of spontaneous erections and urinary continence was 58.1% and 98.4%, respectively.
Lambert et al. [44] announced a cohort of 25 patients treated with focal cryotherapy. PSA failure was defined as nadir + 2 (Phoenix definition) or a decrease in the PSA of less than 50% of the pretreatment PSA value. In the study, with a mean follow-up of 2.3 years, the biochemical-free survival rate was 85%. Erectile function was preserved in 71% of the patients in this study, and no patients experienced worsening of their urinary symptoms.
A study carried out by Ellis et al. [45], with a mean follow-up of 1.3 years, reported on 60 men treated with focal cryotherapy. The biochemical-free survival rate was 80.4%, and the potency rate was 70.6% at the 12-month follow-up. The incontinence rate was 3.6%; yet no patients required the use of absorbent pads for protection.
With a mean follow-up of 3.7 years, Bahn et al. [46] reported on 73 patients treated with focal cryotherapy. Using the ASTRO definition for PSA failure, 75% of patients were free of biochemical recurrence. Potency was maintained in 86% of patients, and 100% of the patients were absent of incontinence. Table 1 shows summary from various studies for focal cryotherapy of prostate.
Even though renal cryotherapy has been studied in animal models, unlike for the prostate, it was not used to treat renal neoplasm until Uchida's publication [16,47]. Now, cryotherapy of small renal masses is expected to replace the conventional partial nephrectomy, being one of the current nephron-sparing techniques. With its advantage of minimizing bleeding and complications, renal cryotherapy has recently been chosen as the alternative to partial nephrectomy for selective small renal masses.
As if to disprove this, the increase in nephron-sparing procedures is outnumbering radical resection (1,424 vs. 1,142), with cryotherapy and RFA taking up about 7.9% of 100,000 patients in 2007 compared with 3.7% in 1998 if looking at the different types of surgery for renal neoplasm that took place in the United States between 1998 and 2008 [48]. The AUA guidelines now clearly list partial nephrectomy as the standard of care for the management of T1a renal tumors. Due to increasing utilization of cross-sectional imaging, nearly 70% of newly detected renal masses are stage T1a, making them more susceptible to minimally invasive nephron-sparing therapies including laparoscopic and robotic partial nephrectomy and ablative therapies [49]. Cryosurgery has developed into a leading option for renal ablation and compared with surgical techniques it offers benefits in preserving renal function with fewer complications, shorter hospitalization, and faster recovery [49]. A mature data set exists at this time, with intermediate and long-term follow-up data available. Cryosurgical recommendations as a first-line therapy are made at this time in limited populations, including old patients, patients with multiple comorbidities, and those with a single kidney.
Acurrent meta-analysis suggested that small renal masses had an average growth rate of 0.3 cm per year, implying that active surveillance is a rational approach in elderly patients with multiple comorbidities who do not wish to continue treatment. In those patients who do wish to pursue treatment, laparoscopic cryoablation (LCA) is a possible minimally invasive alternative [50]. Kunkle et al. [50] analyzed 99 studies representing 6,471 lesions and showed that LCA had a relative risk of 7.45 for local tumor recurrence compared to laparoscopic partial nephrectomy (LPN). Many of these studies used criteria for recurrence established by Weight et al. [51], who demonstrated a high correlation between radiologic and histopathological results, specifically with respect to enhancement on posttreatment MRI. In this analysis, 192 lesions in 176 patients were treated with LCA with a 6-month success rate of 93.8%. Percutaneous biopsy was gained in 97 of these patients. All of the patients who had scans demonstrating enhancement had evidence of residual viable tumor cells on the corresponding biopsy, and none of the biopsies that were performed on treatment sites without enhancement exposed viable tumors, hence demonstrating postcryoablation MRI as a dependable method for screening recurrence. Even though Kunkle's meta-analysis concluded that there was a slightly higher occurrence of residual and recurrent disease with LCA, the progression to metastatic disease did not largely differ, suggesting LCA is still a viable oncologic approach for treating selected small renal masses [50].
The oncologic efficacy of LCA compared with LPN remains the major emphasis in determining the application of this technique. As mentioned above, Kunkle et al. [50] analyzed 99 studies showing 6,471 lesions and concluded that LCA had a relative risk of 7.45 for local tumor recurrence as compared with LPN. A recent meta-analysis by Klatte et al. [52] again showed a 4.82 relative risk of local tumor recurrence following LCA versus LPN in an analysis comparing 5,379 small renal masses treated by LPN and 1,406 small renal masses treated with LCA; in this study, larger tumor size represented the major risk factor for recurrence. Tsivian et al. [53] proved a 4-fold increase in local recurrence with LCA for each 1-cm increase in size.
Aron et al. [54] showed a 92% 5-year disease-specific survival as well as 84% overall survival in 80 patients treated by LCA by a single surgeon. These authors also demonstrated an average of nearly a 90% drop in diameter of the cryoablation zone by 5 years. Complete radiographic disappearance was noted in 73% of the patients in this study. None of the lesions grew, including lesions that had imaging suspicious for local recurrence. A recent single-institution study from Washington University by Tanagho et al. [55] also came up with similar result as Aron's findings, with 80%, 100%, and 76.2% disease-free survival, cancer-specific survival, and overall survival, respectively, with a mean follow-up of 76 months.
Looking at the procedural differences, Desai et al. [56] reported 3 deaths out of the 78 patients treated with cryotherapy but in none of the 153 patients treated with partial nephrectomy; that paper reviewed a database and compared laparoscopic cryotherapy versus LPN. Another matching study carried out by O'Malley et al. [57] reported no recurrence with either treatment, even though the follow-up period was short at less than 12 months. Across database review and matching studies, no differences were found in perioperative outcomes, recovery times, complication rates, or postoperative serum creatinine levels between laparoscopic cryotherapy and LPN. Blood loss was less and surgical time was shorter in the cryotherapy group than in the LPN group.
Ko et al. [58] claimed that there was no local recurrence or metastasis in either group of a matching study that compared laparoscopic cryotherapy and open partial nephrectomy. However, the hospitalized duration was shorter and blood loss was statistically less in the laparoscopic cryotherapy group, whereas no difference was found in the number of patients requiring blood transfusions or in surgical time. The design was limited in that each arm contained only 20 patients and the follow-up period was short. Fig. 2 shows the decreased size of the treated renal cell carcinoma without a definite viable portion postoperatively after renal cryoablation.
Additional advantages of LCA over partial LPN include less blood loss, no need for renal hilar clamping, decreased urine leaks and stenting, and improved access to endophytic tumors. The morbidity benefits of LCA have been described by several authors. Desai et al. [56] demonstrated that partial nephrectomy was associated with more blood loss and delayed complications when compared with LCA. In a meta-analysis comparing complications of LCA versus PN (both open and laparoscopic), investigators found a nearly 10-fold higher relative risk of major complications for PN versus LCA [52]
One of the main advantages of LCA is the conservation of normal renal parenchyma. LCA is an outstanding option for treatment of small renal masses in those patients with chronic renal insufficiency. Tsivian et al. [59] demonstrated that there was no substantial change in chronic renal insufficiency category at any time in 2 years of follow-up of 67 patients after LCA. Tanagho et al. [55] also demonstrated preservation of renal function with moderately stable glomerular filtration rates (GFRs) almost 4 years after LCA (GFR of 68 mL/min preoperatively vs. 65 mL/min postoperatively). These benefits are particularly essential in patients with solitary kidneys and elderly patients with multiple comorbidities.
Another possible use for cryotherapy is in the situation of a tumor in a solitary kidney. Haber et al. [60] examined patients with solitary kidneys who were treated with LPN (n=48) compared with LCA (n=30). Those authors found that estimated GFRs (eGFRs) dropped more with LPN (14.5% vs. 7.3%). Other findings in this study were that LPN was associated with increased blood loss and greater postoperative complications (22.9% vs. 6.7%, p=0.07) compared with LCA. Despite the decreased morbidity with LCA, there were greater recurrence rates in the LCA group (13.3% vs. 0%). The 5-year overall survival was comparable at 93% versus 88%, but there was a significant difference in disease-specific survival (100% in the LPN group vs 88% in the LCA group). The authors concluded that although LCA is technically easier with fewer complications and better eGFR in the long term, LPN showed higher oncologic efficacy. A recent retrospective analysis by Panumatrassame et al. [61] demonstrated that LCA provided better perioperative outcomes with less blood loss, shorter hospitalization, and fewer complications, but the eGFR rates were not meaningfully different between the groups treated with LPN versus LCA.
LPN and LCA affect renal function by possibly distinct mechanisms. With LPN, renal hilar clamping causing renal ischemia and actual parenchymal resection represent two separate mechanisms for potential decreases in eGFR. With LCA, normal parenchymal tissue surrounding the cryolesion may be unintentionally damaged while freezing the tumor [61]. Again there have been varying results reported in the literature. Turna et al. [62] compared three minimally invasive nephron-sparing techniques and concluded that LPN had better intermediate-term oncologic efficacy while having significantly decreased eGFR at 6 months compared with LCA and RFA. Table 2 shows result from various studies for laparoscopic renal cryotherapy.
Many therapies have opened up new optional choices in treating diverse diseases. Cryotherapy is a good example of a treatment method that was largely affected by the development of technology.
Although there are many confounding factors in the biochemical recurrence of prostate cancer, such as how one defines the recurrence and pre- and postoperative hormonal therapy, in the long term, the therapy was encouraging. Moreover, as third-generation cryotherapy has developed, the complication rates have dropped dramatically. Such technological advances have resulted in progress in the use of cryosurgery for the treatment of localized prostate cancer, and recently emerging long-term data have reported favorable results for biochemical recurrence-free survival. Furthermore, many recent studies are actively encouraging further investigation into focal therapies for localized prostate cancer as an alternative to radical treatment or active surveillance.
Despite the fact that LPN should still be considered the gold standard with its superior oncologic efficacy, multiple studies have concluded that LCA is a practical treatment substitute for T1a renal tumors, especially in elderly patients or those with multiple comorbidities who are considered poor candidates for surgery. In order to confirm and clarify the role of cryotherapy in treating prostate cancer and small renal masses, further long-term data must be conducted and more modern techniques and equipment will be required.
Figures and Tables
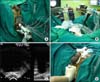 | FIG. 1(A) Ultrathin 17-gauge third-generation cryoneedles were inserted under transrectal ultrasonography guidance, approximately 1 cm apart from the urethra, 5 mm from the prostate capsule and 1 cm from each other. Up to 5 thermosensors were placed midgland, at the level of the external sphincter, left neurovascular bundle, right neurovascular bundle, and Denonvilliers' fascia. (B) Flexible cystoscopy was performed to ensure that none of the needles had inadvertently pierced the urethra. (C) Two freeze-thaw cycles were performed under transrectal ultrasonography guidance. (D) After the cryoneedles were removed, gentle pressure was applied to the perineum for 2 to 5 minutes to minimize bleeding. |
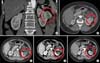 | FIG. 2A 76-year-old female patient with a 3.9-cm left renal cell carcinoma (RCC) had a history of recent acute myocardial infarction, diabetes mellitus, hypertension, and high American Society of Anesthesiologists score. The figure shows the preoperative computed tomography (CT) scan (A and B) and the decreased size of the treated RCC in the left kidney without a definite viable portion at 3 months (C), 9 months (D), and 36 months (E) after left renal cryoablation by abdominal CT scan. |
References
1. Korpan NN. A history of cryosurgery: its development and future. J Am Coll Surg. 2007; 204:314–324.
2. Arnott J. Practical illustrations of the remedial efficacy of a very low or anaesthetic temperature. I. In cancer. Lancet. 1850; 56:257–259.
3. White AC. Liquid air: its application in medicine and surgery. Med Rec. 1899; 56:109–112.
4. Cooper IS, Lee AS, Steininger C. Cryostatic congelation: a system for producing a limited, controlled region of cooling or freezing of biologic tissues. J Nerv Ment Dis. 1961; 133:259–263.
5. Cooper IS. Cryogenic surgery of the basal ganglia. JAMA. 1962; 181:600–604.
6. Gonder MJ, Soanes WA, Smith V. Experimental prostate cryosurgery. Invest Urol. 1964; 1:610–619.
7. Gonder MJ, Soanes WA, Shulman S. Cryosurgical treatment of the prostate. Invest Urol. 1966; 3:372–378.
8. Zacarian SA, Adham MI. Cryotherapy of cutaneous malignancy. Cryobiology. 1966; 2:212–218.
9. Crisp WE, Asadourian L, Romberger W. Application of cryosurgery to gynecologic malignancy. Obstet Gynecol. 1967; 30:668–673.
10. Pandey SK, Milverton EJ, Maloof AJ. A tribute to Charles David Kelman MD: ophthalmologist, inventor and pioneer of phacoemulsification surgery. Clin Experiment Ophthalmol. 2004; 32:529–533.
11. Korpan NN. Basics of cryosurgery. Wien: Springer Verlag Wien;2001.
12. Onik GM, Cohen JK, Reyes GD, Rubinsky B, Chang Z, Baust J. Transrectal ultrasound-guided percutaneous radical cryosurgical ablation of the prostate. Cancer. 1993; 72:1291–1299.
13. Ritch CR, Katz AE. Prostate cryotherapy: current status. Curr Opin Urol. 2009; 19:177–181.
14. Whittaker DK. Mechanisms of tissue destruction following cryosurgery. Ann R Coll Surg Engl. 1984; 66:313–318.
15. Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology. 2002; 60:2 Suppl 1. 40–49.
16. Sindelar WF, Javadpour N, Bagley DH. Histological and ultrastructural changes in rat kidney after cryosurgery. J Surg Oncol. 1981; 18:363–379.
17. Jones JS, Rewcastle JC, Donnelly BJ, Lugnani FM, Pisters LL, Katz AE. Whole gland primary prostate cryoablation: initial results from the cryo on-line data registry. J Urol. 2008; 180:554–558.
18. Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008; 148:435–448.
19. Chin JL, Ng CK, Touma NJ, Pus NJ, Hardie R, Abdelhady M, et al. Randomized trial comparing cryoablation and external beam radiotherapy for T2C-T3B prostate cancer. Prostate Cancer Prostatic Dis. 2008; 11:40–45.
20. Mouraviev V, Polascik TJ. Update on cryotherapy for prostate cancer in 2006. Curr Opin Urol. 2006; 16:152–156.
21. Cohen JK, Miller RJ Jr, Ahmed S, Lotz MJ, Baust J. Ten-year biochemical disease control for patients with prostate cancer treated with cryosurgery as primary therapy. Urology. 2008; 71:515–518.
22. Han KR, Cohen JK, Miller RJ, Pantuck AJ, Freitas DG, Cuevas CA, et al. Treatment of organ confined prostate cancer with third generation cryosurgery: preliminary multicenter experience. J Urol. 2003; 170(4 Pt 1):1126–1130.
23. Hubosky SG, Fabrizio MD, Schellhammer PF, Barone BB, Tepera CM, Given RW. Single center experience with third-generation cryosurgery for management of organ-confined prostate cancer: critical evaluation of short-term outcomes, complications, and patient quality of life. J Endourol. 2007; 21:1521–1531.
24. De Meerleer GO, Fonteyne VH, Vakaet L, Villeirs GM, Denoyette L, Verbaeys A, et al. Intensity-modulated radiation therapy for prostate cancer: late morbidity and results on biochemical control. Radiother Oncol. 2007; 82:160–166.
25. Zapatero A, Minguez R, Nieto S, Martin de, Garcia-Vicente F. Post-treatment prostate biopsies in the era of threedimensional conformal radiotherapy: what can they teach us? Eur Urol. 2009; 55:902–909.
26. Stephenson AJ, Scardino PT, Bianco FJ Jr, DiBlasio CJ, Fearn PA, Eastham JA. Morbidity and functional outcomes of salvage radical prostatectomy for locally recurrent prostate cancer after radiation therapy. J Urol. 2004; 172(6 Pt 1):2239–2243.
27. Izawa JI, Perrotte P, Greene GF, Scott S, Levy L, McGuire E, et al. Local tumor control with salvage cryotherapy for locally recurrent prostate cancer after external beam radiotherapy. J Urol. 2001; 165:867–870.
28. Pisters LL, Rewcastle JC, Donnelly BJ, Lugnani FM, Katz AE, Jones JS. Salvage prostate cryoablation: initial results from the cryo on-line data registry. J Urol. 2008; 180:559–563.
29. Chin JL, Touma N, Pautler SE, Guram KS, Bella AJ, Downey DB, et al. Serial histopathology results of salvage cryoablation for prostate cancer after radiation failure. J Urol. 2003; 170(4 Pt 1):1199–1202.
30. Izawa JI, Busby JE, Morganstern N, Vakar-Lopez F, Scott SM, Pisters LL. Histological changes in prostate biopsies after salvage cryotherapy: effect of chronology and the method of biopsy. BJU Int. 2006; 98:554–558.
31. Ng CK, Moussa M, Downey DB, Chin JL. Salvage cryoablation of the prostate: followup and analysis of predictive factors for outcome. J Urol. 2007; 178(4 Pt 1):1253–1257.
32. Ghafar MA, Johnson CW, De La, Benson MC, Bagiella E, Fatal M, et al. Salvage cryotherapy using an argon based system for locally recurrent prostate cancer after radiation therapy: the Columbia experience. J Urol. 2001; 166:1333–1337.
33. Ismail M, Ahmed S, Kastner C, Davies J. Salvage cryotherapy for recurrent prostate cancer after radiation failure: a prospective case series of the first 100 patients. BJU Int. 2007; 100:760–764.
34. Long JP, Fallick ML, LaRock DR, Rand W. Preliminary outcomes following cryosurgical ablation of the prostate in patients with clinically localized prostate carcinoma. J Urol. 1998; 159:477–484.
35. Han KR, Belldegrun AS. Third-generation cryosurgery for primary and recurrent prostate cancer. BJU Int. 2004; 93:14–18.
36. Anastasiadis AG, Sachdev R, Salomon L, Ghafar MA, Stisser BC, Shabsigh R, et al. Comparison of health-related quality of life and prostate-associated symptoms after primary and salvage cryotherapy for prostate cancer. J Cancer Res Clin Oncol. 2003; 129:676–682.
37. Babaian RJ, Donnelly B, Bahn D, Baust JG, Dineen M, Ellis D, et al. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol. 2008; 180:1993–2004.
38. Onik G, Vaughan D, Lotenfoe R, Dineen M, Brady J. The "male lumpectomy": focal therapy for prostate cancer using cryoablation results in 48 patients with at least 2-year follow-up. Urol Oncol. 2008; 26:500–505.
39. Abern MR, Tsivian M, Polascik TJ. Focal therapy of prostate cancer: evidence-based analysis for modern selection criteria. Curr Urol Rep. 2012; 13:160–169.
40. Bomers JG, Sedelaar JP, Barentsz JO, Futterer JJ. MRI-guided interventions for the treatment of prostate cancer. AJR Am J Roentgenol. 2012; 199:714–720.
41. Bozzini G, Colin P, Nevoux P, Villers A, Mordon S, Betrouni N. Focal therapy of prostate cancer: energies and procedures. Urol Oncol. 2013; 31:155–167.
42. Onik G, Narayan P, Vaughan D, Dineen M, Brunelle R. Focal "nerve-sparing" cryosurgery for treatment of primary prostate cancer: a new approach to preserving potency. Urology. 2002; 60:109–114.
43. Ward JF, Jones JS. Focal cryotherapy for localized prostate cancer: a report from the national Cryo On-Line Database (COLD) Registry. BJU Int. 2012; 109:1648–1654.
44. Lambert EH, Bolte K, Masson P, Katz AE. Focal cryosurgery: encouraging health outcomes for unifocal prostate cancer. Urology. 2007; 69:1117–1120.
45. Ellis DS, Manny TB Jr, Rewcastle JC. Focal cryosurgery followed by penile rehabilitation as primary treatment for localized prostate cancer: initial results. Urology. 2007; 70:6 Suppl. 9–15.
46. Bahn D, de Castro Abreu AL, Gill IS, Hung AJ, Silverman P, Gross ME, et al. Focal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 years. Eur Urol. 2012; 62:55–63.
47. Uchida M, Imaide Y, Sugimoto K, Uehara H, Watanabe H. Percutaneous cryosurgery for renal tumours. Br J Urol. 1995; 75:132–136.
48. Woldrich JM, Palazzi K, Stroup SP, Sur RL, Parsons JK, Chang D, et al. Trends in the surgical management of localized renal masses: thermal ablation, partial and radical nephrectomy in the USA, 1998-2008. BJU Int. 2013; 111:1261–1268.
49. Berger A, Kamoi K, Gill IS, Aron M. Cryoablation for renal tumors: current status. Curr Opin Urol. 2009; 19:138–142.
50. Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma--a meta-analysis and review. J Urol. 2008; 179:1227–1233.
51. Weight CJ, Kaouk JH, Hegarty NJ, Remer EM, O'Malley CM, Lane BR, et al. Correlation of radiographic imaging and histopathology following cryoablation and radio frequency ablation for renal tumors. J Urol. 2008; 179:1277–1281.
52. Klatte T, Grubmuller B, Waldert M, Weibl P, Remzi M. Laparoscopic cryoablation versus partial nephrectomy for the treatment of small renal masses: systematic review and cumulative analysis of observational studies. Eur Urol. 2011; 60:435–443.
53. Tsivian M, Lyne JC, Mayes JM, Mouraviev V, Kimura M, Polascik TJ. Tumor size and endophytic growth pattern affect recurrence rates after laparoscopic renal cryoablation. Urology. 2010; 75:307–310.
54. Aron M, Kamoi K, Remer E, Berger A, Desai M, Gill I. Laparoscopic renal cryoablation: 8-year, single surgeon outcomes. J Urol. 2010; 183:889–895.
55. Tanagho YS, Roytman TM, Bhayani SB, Kim EH, Benway BM, Gardner MW, et al. Laparoscopic cryoablation of renal masses: single-center long-term experience. Urology. 2012; 80:307–314.
56. Desai MM, Aron M, Gill IS. Laparoscopic partial nephrectomy versus laparoscopic cryoablation for the small renal tumor. Urology. 2005; 66:5 Suppl. 23–28.
57. O'Malley RL, Berger AD, Kanofsky JA, Phillips CK, Stifelman M, Taneja SS. A matched-cohort comparison of laparoscopic cryoablation and laparoscopic partial nephrectomy for treating renal masses. BJU Int. 2007; 99:395–398.
58. Ko YH, Park HS, Moon du G, Lee JG, Kim JJ, Yoon DK, et al. A matched-cohort comparison of laparoscopic renal cryoablation using ultra-thin cryoprobes with open partial nephrectomy for the treatment of small renal cell carcinoma. Cancer Res Treat. 2008; 40:184–189.
59. Tsivian M, Caso J, Kimura M, Polascik TJ. Renal function outcomes after laparoscopic renal cryoablation. J Endourol. 2011; 25:1287–1291.
60. Haber GP, Lee MC, Crouzet S, Kamoi K, Gill IS. Tumour in solitary kidney: laparoscopic partial nephrectomy vs laparoscopic cryoablation. BJU Int. 2012; 109:118–124.
61. Panumatrassamee K, Kaouk JH, Autorino R, Lenis AT, Laydner H, Isac W, et al. Cryoablation versus minimally invasive partial nephrectomy for small renal masses in the solitary kidney: impact of approach on functional outcomes. J Urol. 2013; 189:818–822.
62. Turna B, Kaouk JH, Frota R, Stein RJ, Kamoi K, Gill IS, et al. Minimally invasive nephron sparing management for renal tumors in solitary kidneys. J Urol. 2009; 182:2150–2157.
63. Wyler SF, Sulser T, Ruszat R, Weltzien B, Forster TH, Provenzano M, et al. Intermediate-term results of retroperitoneoscopy-assisted cryotherapy for small renal tumours using multiple ultrathin cryoprobes. Eur Urol. 2007; 51:971–979.
64. Weld KJ, Figenshau RS, Venkatesh R, Bhayani SB, Ames CD, Clayman RV, et al. Laparoscopic cryoablation for small renal masses: three-year follow-up. Urology. 2007; 69:448–451.
65. Wright AD, Turk TM, Nagar MS, Phelan MW, Perry KT. Endophytic lesions: a predictor of failure in laparoscopic renal cryoablation. J Endourol. 2007; 21:1493–1496.
66. Derweesh IH, Malcolm JB, Diblasio CJ, Giem A, Rewcastle JC, Wake RW, et al. Single center comparison of laparoscopic cryoablation and CT-guided percutaneous cryoablation for renal tumors. J Endourol. 2008; 22:2461–2467.
67. Ko YH, Choi H, Kang SG, Park HS, Lee JG, Kim JJ, et al. Efficacy of laparoscopic renal cryoablation as an alternative treatment for small renal mass in patients with poor operability: experience from the Korean single center. J Laparoendosc Adv Surg Tech A. 2010; 20:339–345.
68. Guazzoni G, Cestari A, Buffi N, Lughezzani G, Nava L, Cardone G, et al. Oncologic results of laparoscopic renal cryoablation for clinical T1a tumors: 8 years of experience in a single institution. Urology. 2010; 76:624–629.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download