1. Juan YS, Chuang SM, Jang MY, Huang CH, Chou YH, Wu WJ, et al. Basic research in bladder outlet obstruction. Incont Pelvic Floor dysfunct. 2011; 5:1–6.
2. Tucci S Jr, Molina CA, Cassini MF, Andrade MF, Lima GJ, Martins AC. Chronic partial urethral obstruction in female rats: description of an experimental model and initial results. Acta Cir Bras. 2011; 26(Suppl 2):111–114. PMID:
22030825.
3. Peters CA. Congenital bladder obstruction: research strategies and directions. Adv Exp Med Biol. 1995; 385:117–130. PMID:
8571823.

4. Levin RM, Chichester P, Hass MA, Gosling JA, Buttyan R. Obstructive bladder dysfunction: morphological, biochemical and molecular changes. Eur Urol Suppl. 2002; 1:14–20.

5. Tseng TY, Stoller ML. Obstructive uropathy. Clin Geriatr Med. 2009; 25:437–443. PMID:
19765491.

6. Hanai T, Ma FH, Matsumoto S, Park YC, Kurita T. Partial outlet obstruction of the rat bladder induces a stimulatory response on proliferation of the bladder smooth muscle cells. Int Urol Nephrol. 2002; 34:37–42. PMID:
12549637.
7. Parkhouse HF, Barratt TM, Dillon MJ, Duffy PG, Fay J, Ransley PG, et al. Long-term outcome of boys with posterior urethral valves. Br J Urol. 1988; 62:59–62. PMID:
3408870.

8. Dedeoglu F, Rose CD, Athreya BH, Conard K, Grissom L, Magnusson M. Successful treatment of retroperitoneal fibrosis with tamoxifen in a child. J Rheumatol. 2001; 28:1693–1695. PMID:
11469481.
9. Delle H, Rocha JR, Cavaglieri RC, Vieira JM Jr, Malheiros DM, Noronha IL. Antifibrotic effect of tamoxifen in a model of progressive renal disease. J Am Soc Nephrol. 2012; 23:37–48. PMID:
22052053.
10. Shirazi M, Noorafshan A, Bahri MA, Hassanpoor A. Captopril reduces deposition of collagen in lamina propria and muscular layers of the bladder and ureter in neonatal dogs with partial urethral obstruction. Scand J Urol Nephrol. 2008; 42:324–329. PMID:
19230165.

11. Akbar DH, Hagras MM, Amin HA, Khorshid OA. Comparison between the effect of glibenclamide and captopril on experimentally induced diabetic nephropathy in rats. J Renin Angiotensin Aldosterone Syst. 2013; 14:103–115. PMID:
23077081.

12. Shirazi M, Noorafshan A, Farrokhi A. Effects of pentoxifylline on renal structure after urethral obstruction in rat: a stereological study. Cent European J Urol. 2011; 64:30–33.

13. Zhou QG, Zheng FL, Hou FF. Inhibition of tubulointerstitial fibrosis by pentoxifylline is associated with improvement of vascular endothelial growth factor expression. Acta Pharmacol Sin. 2009; 30:98–106. PMID:
19079293.

14. Acikgoz Y, Can B, Bek K, Acikgoz A, Ozkaya O, Genc G, et al. The effect of simvastatin and erythropoietin on renal fibrosis in rats with unilateral ureteral obstruction. Ren Fail. 2014; 36:252–257. PMID:
24083846.

15. Vieira JM Jr, Mantovani E, Rodrigues LT, Delle H, Noronha IL, Fujihara CK, et al. Simvastatin attenuates renal inflammation, tubular transdifferentiation and interstitial fibrosis in rats with unilateral ureteral obstruction. Nephrol Dial Transplant. 2005; 20:1582–1591. PMID:
15855201.
16. Weibel ER, Hsia CC, Ochs M. How much is there really? Why stereology is essential in lung morphometry. J Appl Physiol (1985). 2007; 102:459–467. PMID:
16973815.

17. Bajpai M, Pratap A, Tripathi M, Bal CS. Posterior urethral valves: preliminary observations on the significance of plasma Renin activity as a prognostic marker. J Urol. 2005; 173:592–594. PMID:
15643266.

18. Chen YM, Chien CT, Hu-Tsai MI, Wu KD, Tsai CC, Wu MS, et al. Pentoxifylline attenuates experimental mesangial proliferative glomerulonephritis. Kidney Int. 1999; 56:932–943. PMID:
10469361.

19. Nyengaard JR. Stereologic methods and their application in kidney research. J Am Soc Nephrol. 1999; 10:1100–1123. PMID:
10232698.

20. Karbalay-Doust S, Noorafshan A, Pourshahid SM. Taxol and taurine protect the renal tissue of rats after unilateral ureteral obstruction: a stereological survey. Korean J Urol. 2012; 53:360–367. PMID:
22670197.

21. von Bartheld CS. Distribution of particles in the z-axis of tissue sections: relevance for counting methods. Neuroquantology. 2012; 10:66–75. PMID:
23874137.

22. Egido J, Gomez-Chiarri M, Ortiz A, Bustos C, Alonso J, Gomez-Guerrero C, et al. Role of tumor necrosis factor-alpha in the pathogenesis of glomerular diseases. Kidney Int Suppl. 1993; 39:S59–S64. PMID:
8385721.
23. Shirazi M, Noorafshan A, Bahri MA, Tanideh N. Captopril reduces interstitial renal fibrosis and preserves more normal renal tubules in neonatal dogs with partial urethral obstruction: a preliminary study. Urol Int. 2007; 78:173–177. PMID:
17293660.

24. Shirazi M, Noorafshan A, Kroup M, Tanideh N. Comparison of the effects of captopril, tamoxifen and L-carnitine on renal structure and fibrosis after total unilateral ureteral obstruction in the rat. Scand J Urol Nephrol. 2007; 41:91–97. PMID:
17454945.

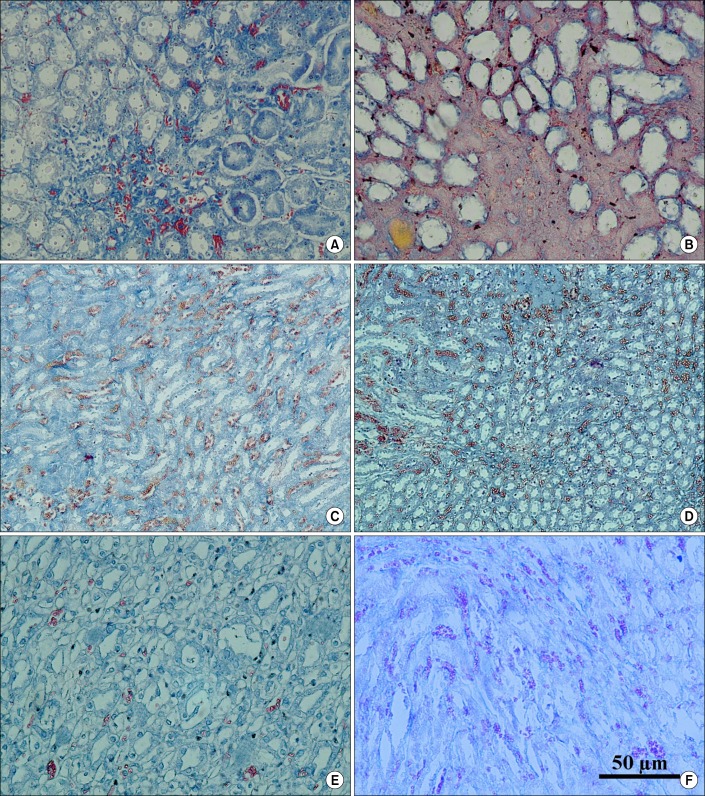
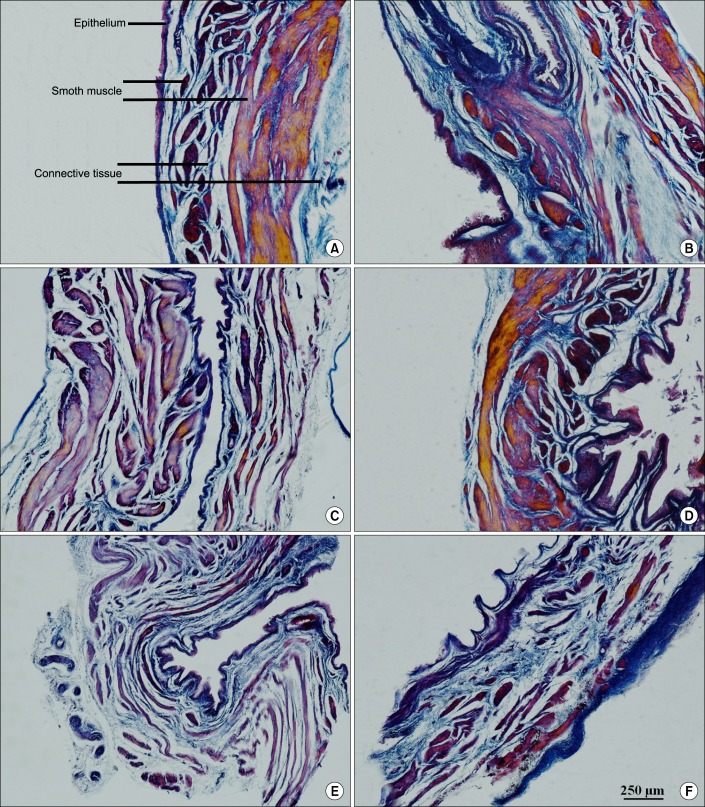
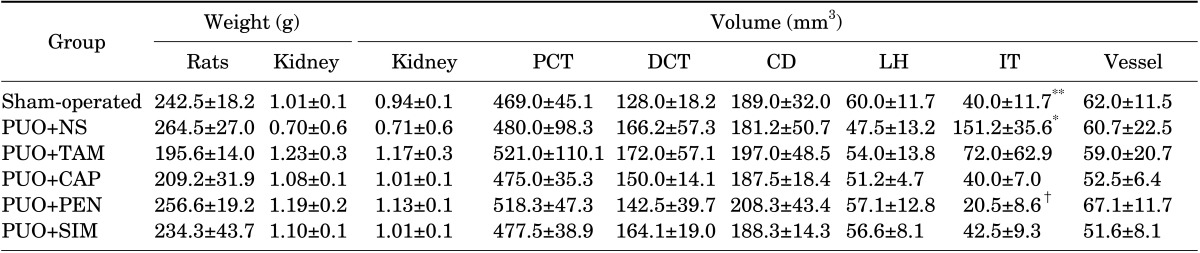
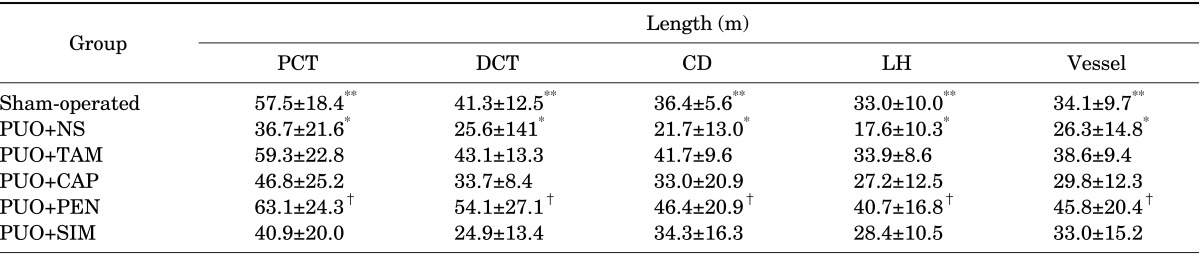




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download