Abstract
Purpose
To investigate the effects of estrogen on the expression of the α1 receptor and nitric oxide synthase (NOS) in rat urethra and bladder after oophorectomy.
Materials and Methods
Forty-five mature female Sprague-Dawley rats (aged 10-11 weeks, 235-250 g) were randomly assigned to one of three groups: control group, oophorectomy group (Opx), or oophorectomy and estradiol replacement group (Opx+ Est). The degree of expression of α1 receptor (α1A and D) and NOS (neuronal NOS [nNOS] and endothelial NOS [eNOS]) in bladder and urethral tissues was investigated by using immunohistochemical staining and Western blotting.
Results
In the bladder, the expression rates of α1 receptor (α1A and α1D) increased in the Opx group but decreased in the Opx+Est group. These changes were not statistically significant. The α1A and α1D receptor of the urethra decreased in the Opx group but increased in the Opx+Est group. These changes were not statistically significant. In the bladder and urethra, the expression rates of nNOS and eNOS significantly increased in the Opx group but decreased in the Opx+Est group (p<0.05).
Menopause causes numerous physical changes in women. In particular, marked changes take place in urination symptoms, including the possibility of urinary incontinence, frequency, and urgency. Furthermore, when estrogen is supplemented to women during menopause, the increase in the internal pressure of the urethra, the transfer of intra-abdominal pressure to the proximal urethra, and the sensory threshold of the bladder are mechanisms by which urinary incontinence improves [1].
The bladder reflex relies mostly on the efferent and afferent neurotransmission of the pelvic nerve. The efferent nerve of the bladder through the pelvic nerve is mostly cholinergic, and adrenergic neurotransmission regulates its activities. The hypogastric nerve is the main adrenergic nerve of the bladder and the pelvic nerve is the additional path [2]. Two key adrenergic receptors, namely, the α and β receptors, were discovered in the bladder. Of these two, the α1 receptor functions mostly to regulate the contraction of the bladder, whereas the α2 and the β receptors can relax the bladder muscles [3,4]. Among the α1 receptors, α1A and α1D are distributed mostly in the bladder [5]. Moreover, atrophy of the nonadrenergic nerve of the bladder results from either oophorectomy or aging, which in turn can aggravate the changes in the adrenergic receptor [5]. That is, symptoms of overactive bladder including frequency and urgency following menopause can be affected by the adrenergic receptor.
Recent research showed that nitric oxide (NO) is excreted by the nonadrenergic and noncholinergic (NANC) nerves in various mammals [6,7,8], and it was proven that estrogen regulates the activities of the nitric oxide synthase (NOS) that generates NO in various tissues [9]. Many researchers have pursued studies on the neurotransmitters and tissue changes involved in the actions of estrogen. The present study is a continuation of such studies.
The present study aimed to examine the changes in the NO excreted at the α1 receptor and NANC nerve terminal in the bladder and urethra in white rats (Sprague Dawley) that have undergone oophorectomy and supplementation with estrogen. The analyses were carried out by immunohistochemical study and Western blot. In addition, changes in the epithelial cells, muscle layer, and collagen in the bladder and urethra were examined to study associations between them.
Forty-five white rats (Sprague Dawley) aged 10 to 11 weeks with a body weight range of 235 to 250 g were used as the experimental animals. The animals were divided into the control group (Con group, in which sham surgery only was performed, n=15), the oophorectomy group (Opx group, n=15), and the hormone-supplemented group oophorectomy group (Opx+Est group, n=15). All rats were raised under the same conditions. All animal studies were performed after obtaining approval from the ethics committee and in accordance with the animal experimentation guidelines.
All experimental animals were anesthetized with ketamine (25 mg/kg) and xylazine (5 mg/kg) injected into the abdominal cavity, after which the abdomen was opened by a midline incision. Bilateral oophorectomy was performed on the Opx group, whereas only sham surgery was performed on the Con group. The Opx+Est group underwent injection of estradiol cypionate (0.25 mg/1 wk, Miro-depot injection, CJ Hanil Pharmaceutical, Seoul, Korea) into the muscle 2 weeks after oophorectomy. In addition, the Opx group was injected with sesame oil only (0.25 mL) in the same manner (Table 1).
Approximately 5 mL of blood was collected through the aorta at the time of tissue collection for measurement of the 17β-estradiol level by use of DPC's Coat-A-Count RIA kit (Diagnostic Products Co., Los Angeles, CA, USA). The concentration of 17β-estradiol was 3.47±2.42 pg/mL in the Opx group, which was significantly less than the concentration in the Con group of 16.27±15.49 pg/mL, whereas the Opx+Est group displayed a significantly higher concentration of 28.68±10.51 pg/mL (p<0.05) (Table 2).
Tissues were collected after the experimental animals were anesthetized by injection of ketamine (25 mg/kg) and xylazine (5 mg/kg) into the abdominal cavity 2 weeks after oophorectomy in the Opx group and 2 weeks after hormone supplementation, which was performed 2 weeks after oophorectomy, in the Opx+Est group (Table 1). For the tissues to be used for immunohistochemical staining, the surrounding fatty tissues and connective tissues were removed and cleaned with physiological saline solution before being fixed in 10% formalin. Tissues to be used for Western blot were immediately frozen for storage by using liquefied nitrogen.
Paraffin-embedded bladder and urethra tissues were sliced at a thickness of 4 µm and were subjected to immunohistochemical staining. For tissues that had undergone a dehydration procedure, tissues to be used for eNOS staining were fixed after having been boiled for 15 minutes by immersing them in citrate buffer and using an autoclave. And tissues to be used for nNOS staining were fixed immediately. Then, the primary antibody was reacted with the prepared tissues. For nNOS, rabbit polyclonal anti-nNOS antibody (Zymed Laboratories, San Francisco, CA, USA) was diluted at a ratio of 1:250; for eNOS, rabbit polyclonal anti-eNOS antibody (Lab Vision Inc., Fremont, CA, USA) was diluted at a ratio of 1:200. Both antibodies were allowed to react overnight at 4℃ before being subjected to the secondary antibody to which biotin was attached for 30 minutes. Both were then cleansed in phosphate buffered saline three times for 5 minutes each before reaction with streptavidin for 30 minutes, after which the samples were cleansed three times for 5 minutes each again. Color was then allowed to develop with amino-ethylcarbazole.
Tissues were immersed in 500 µL of lysis buffer (1M Tris-Cl, 0.5M NaCl, 0.5M ethylenediaminetetraacetic acid) to which pepstatin (0.7 mg/mL), a proteolytic enzyme inhibitor, was added and were allowed to react for 30 minutes at 4℃ to dissolve the cells. The mixture was centrifuged for 15 minutes at 14,000 rpm and the supernatant was separated and the protein was quantified. A total of 20 µg of protein was loaded into each lane of a 12% sodium dodecyl sulfate-polyacrylamide gel (Novex, Wadsworth, OH, USA) for electrophoresis. The gel was then transferred to a 0.45-µm Westran membrane (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and all antibody reactions were carried out in the nonspecific blocking buffer (5% skim milk in 1% Tween-20/Tris buffered saline). The membrane to which protein was transferred was first immersed in blocking buffer and left to react for 1 to 2 hours at room temperature. Then, it was left to react again for 1 hour at 37℃ with primary antibody for eNOS (Santa Cruz Biotechnology, Dallas, TX, USA), primary antibody for nNOS (Santa Cruz Biotechnology), primary antibody for α1A (Santa Cruz Biotechnology), primary antibody for α1D (Santa Cruz Biotechnology), and primary antibody for actin (Santa Cruz Biotechnology), respectively, all of which were diluted at a ratio of 1:1000. Bands were visualized by chemiluminescence (Amersham Pharmacia Biotech, Little Chalfont, UK); actin was presented as the standard value. A Star Scanner using NIH image V1-57 software was used for densitometry analysis.
Observation of changes in smooth muscle and collagen contents: To examine the changes in the relative compositional ratio of the smooth muscle component and collagen component of bladder and urethra, the paraffin-embedded tissues were sliced at a thickness of 4 µm and subjected to Masson's trichrome staining. Ten viewing spots under ×400 magnification were selected randomly and the number of collagen cells observed within the chosen lattice was converted into a percentage and averaged.
Changes in estrogen concentrations were comparatively analyzed by using Mann-Whitney U tests, whereas changes in α1 receptor, NOS, and smooth muscle and collagen contents in the 3 groups were comparatively analyzed by using the Kruskall-Wallis test. The changes were determined to be statistically significant if the p-value was less than 0.05.
The expression of α1A receptor protein in bladder tissue showed a tendency to increase after oophorectomy, whereas it displayed a tendency to decrease after estrogen supplementation. However, such changes were not statistically significant (Fig. 1).
The expression of α1D receptor protein in bladder tissue showed a tendency to increase after oophorectomy, whereas it displayed a tendency to decrease after estrogen supplementation. However, such changes were not statistically significant (Fig. 2).
The expression of neuronal nitric oxide synthase (nNOS) was most marked in the smooth muscle of the bladder in all groups. Although the expression of nNOS in the mucous membrane and smooth muscle of the bladder was similar in the groups, in the blood vessels, the expression of nNOS was higher in the Opx group than in the Con group and was lower in the Opx+Est group than in the Opx group (Fig. 3).
The expression of nNOS protein in bladder tissue increased significantly after oophorectomy and decreased after estrogen supplementation to an extent similar to that in the Con group. The Con, Opx, and Opx+Est groups displayed significant differences (Fig. 4).
The expression of endothelial nitric oxide synthase (eNOS) in the bladder was particularly marked in the mucous membrane, and it was also found in smooth muscle and blood vessels. Overall, the expression of eNOS was higher in the Opx group than in the Con group, whereas it was lower in the Opx+Est group than in the Opx group (Fig. 5).
The expression of eNOS protein in bladder tissue increased significantly after oophorectomy and decreased after estrogen supplementation to an extent similar to that of the Con group. The Con, Opx, and Opx+Est groups displayed significant differences, respectively (Fig. 6).
In the histological study, a reduction in the distribution of blood vessels, atrophy of epithelial cells, and a reduction in the muscle layer of the bladder were observed in the Opx group. By contrast, an increase in the distribution of blood vessels, proliferation of epithelial cells, and an increase in the muscle layer of the bladder were observed in the Opx+Est group (Fig. 7). Examination of the changes in the smooth muscle and collagen components by means of Masson's trichrome staining revealed that the relative compositional ratio of collagen in the bladder increased in the Opx group, whereas the Opx+Est group demonstrated a significant reduction in comparison with the Opx group but no significant difference compared with the Con group.
The expression of the α1A receptor protein in the urethra tissue tended to increase after oophorectomy and tended to decrease after estrogen supplementation. However, these changes were not statistically significant (Fig. 8).
The expression of the α1D receptor protein in the urethra tissue tended to increase after oophorectomy and tended to decrease after estrogen supplementation. However, these changes were not statistically significant (Fig. 9).
The expression of nNOS increased in the mucous membrane, smooth muscle, and blood vessels of the urethra in the Opx group compared with the Con group, whereas nNOS expression decreased in the Opx+Est group compared with the Opx group (Fig. 10).
The expression of nNOS protein in urethra tissues increased significantly after oophorectomy and decreased after estrogen supplementation to an extent similar to that in the Con group. Each of the groups demonstrated significant differences (Fig. 11).
The expression of eNOS increased in the mucous membranes, smooth muscle, and blood vessels of the urethra in the Opx group compared with the Con group, whereas it decreased in the Opx+Est group compared with the Opx group (Fig. 12).
The expression of eNOS protein in urethra tissues increased significantly after oophorectomy and decreased after estrogen supplementation to an extent similar to that in the Con group. The Con, Opx, and Opx+Est groups displayed significant differences, respectively (Fig. 13).
In the histological study, a reduction in the distribution of blood vessels, atrophy of epithelial cells, and a reduction in the muscle layer of the urethra were observed for the Opx group, whereas an increase in the distribution of blood vessels, proliferation of epithelial cells, and an increase in the muscle layer of the urethra were observed in the Opx+Est group (Fig. 14). Examination of the changes in the smooth muscle and collagen components by means of Masson's trichrome staining revealed that the relative compositional ratio of the collagen in the urethra increased in the Opx group, whereas that of the Opx+Est group demonstrated a significant reduction compared with the Opx group but showed no significant difference compared with the Con group.
The main symptoms of the lower urinary tract, including frequency, urgency, and urinary incontinence, are found in menopausal women, and these symptoms result from changes in the lower urinary tract due to the concentration of estrogen and due to changes in various nerve systems and bladder and urethral tissues [10,11]. Hextall and Cardozo [12] asserted that the roles of estrogen in the lower urinary tract include, first, activating the cellular cycle in the bladder and urethra; second, increasing the blood flow rate in the mucous membrane and sphincter muscle of the urethra; third, sensitizing the α-adrenergic receptor of the urethra; and fourth, preventing aging by stimulating the metabolism of connective tissues surrounding the urethra. However, estrogen displays its effects through indirect actions on the concentration and sensitivity of the receptors of various hormones and neurotransmitters and on pharmacological interactions such as the metabolism of neurotransmitters in the lower urinary tract rather than by direct action through the receptor [10]. Therefore, although it is theoretically anticipated that estrogen supplementation therapy could improve the urination symptoms of menopausal women, the reality is that such therapy fails to achieve significant clinical effectiveness [13,14]. Accordingly, numerous researchers have been pursuing studies of the changes that take place in the neurotransmitters and tissues involved in the actions of estrogen. Reaction of the bladder and urethra by adrenergic stimulation through the α1 receptor and reaction of the urethra by stimulation of the NANC nerve are known to mediate the action of estrogen on the lower urinary tract in women [1,15]. It is said that there is an increase in the distribution of blood vessels, proliferation of epithelial cells, and in the muscle layer and an increase or reduction in collagen in the bladder and urethra owing to such action of estrogen [11].
The present study examined changes in the NO excreted at the α1 receptor and NANC nerve terminal in the bladder and urethra in white rats that had undergone oophorectomy and supplementation with estrogen. In addition, changes in the epithelial cells, muscle layer, and collagen in the bladder and urethra were examined to uncover clues to the relevance between them.
The α1 receptors in the bladder increase after menopause and can in turn increase the contractibility of the bladder, thereby increasing the possibility of frequency and urgency during menopause. On the other hand, the contraction of the urethra is known to decrease by adrenergic stimulation through α1 receptors in the urethra following menopause, thereby aggravating the symptoms of urinary incontinence [5]. Although it was asserted in several studies that the α receptor agonist fenofibrate is effective when concurrently administrated with phenylpropanolamine, which is an α-adrenergic agonist, and can be applied usefully in the treatment of urinary incontinence patients with intra-abdominal pressure for whom surgery cannot be applied, there in fact appear to be limitations in application owing to side effects on the blood vessels of the brain. Currently, drugs such as ephedrine or pseudoephedrine can be applied for such purpose [15]. In this experiment, the white rat models in which the estrogen was supplemented following oophorectomy also displayed results that were in concordance to a certain extent, although they were not statistically significant. Although the α receptors of the urethra of white rats decreased after oophorectomy, the number of receptors increased again through estrogen supplementation. Similar results were found in research by Dmitrieva [16], which showed that greater improvement in lower urinary tract symptoms could be achieved by concurrently administering the α receptor agonist fenofibrate at the time of estrogen supplementation. It is deemed that the changes in the α receptor in accordance with hormone supplementation in this study were not statistically significant because of the short period of supplementation and failure to accurately distinguish the subtypes of α receptor owing to difficulties in distinguishing the bladder and urethra of white rats.
The relaxation response of the urethra resulting from stimulation of the NANC nerve was another key subject of this study. NO is secreted from the NANC nerve [17], which can cause relaxation of the urethra in white rats [18], and this phenomenon was proven in the human body [8]. Takahashi et al. [1] demonstrated the reduction in NOS in the urethra of rabbits at the time of hormone supplementation therapy and asserted that this inhibits the relaxation of the urethra through NANC stimulation by reducing NO. Fractionation of NOS includes eNOS, nNOS, and iNOS, which regulate the contraction of the urethra in the tissues of the lower urinary tract without inflammation, with eNOS and nNOS playing a key role in the concentration of NO [19]. In this study, nNOS was distributed mostly in the smooth muscles of the bladder and the urethra, whereas eNOS displayed a high concentration in the mucous membrane of the bladder as well as in the blood vessels. Moreover, the expression of nNOS and eNOS increased significantly within the urethral tissues of white rates subjected to oophorectomy but decreased at the time of hormone supplementation. Okuno et al. [20] asserted in their research using rabbits that NO within the tissues of the urethra increased at the time of oophorectomy and decreased at the time of estrogen supplementation, thereby inhibiting relaxation of the urethra.
It was possible to confirm in this study that the contractibility and elasticity of the urethra decrease owing to an increase in the relaxation of the urethra by NO and that the conditions for urinary incontinence are generated as a result of the atrophy of the urethra after menopause.
This study had the limitation of a difference between the results of the experiment and those predicted in the research plan from the perspective that the number of experimental animals was limited and larger animals such as rabbits could not be used. More statistically significant results could be obtained if the experiment could be carried out by definitively segregating the bladder and the urethra, and the mucous membrane and smooth muscle, by using a larger number of experimental animals and larger animals.
Figures and Tables
 | FIG. 1α1A receptor protein expression in the rat bladder. (A) Protein is extracted from the bladder in the Con, Opx, and Opx+Est group, and it is analyzed for the α1A receptor and actin levels by Western blotting. (B) The relative amount of α1A receptor protein increased after oophorectomy. It is normalized after Estradiol replacement. However, the expressions of α1A receptor protein in the Con, Opx, and Opx+Est group are not significantly different. Con, control; Opx, oophorectomy; Opx+ Est, 17β-estradiol replacement after oophrorectomy. |
 | FIG. 2α1D receptor protein expression in the rat bladder. (A) Protein is extracted from the bladder in the Con, Opx, and Opx+Est group, and it is analyzed for the α1D receptor and actin levels by Western blotting. (B) The relative amount of α1D receptor protein increased after oophorectomy. It is normalized after Estradiol replacement. However, the expressions of α1D receptor protein in the Con, Opx, and Opx+Est group are not significantly different. Con, control; Opx, oophorectomy; Opx+Est, 17β-estradiol replacement after oophrorectomy. |
 | FIG. 3Immunreactivity pattern for neuronal nitric oxide synthase (nNOS) of rat bladder (immunohistochemical stain, ×400). The blood vessel, the expression of nNOS increased in the Opx group in comparison to the Con group (A), and decreased in the Opx+Est group (B) in comparison to the Opx group (C). Con, control; Opx, oophorectomy; Opx+Est, 17β-estradiol replacement after oophrorectomy. |
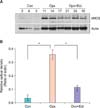 | FIG. 4Neuronal nitric oxide synthase (nNOS) protein expression in the rat bladder. (A) Protein is extracted from the bladder in the Con, Opx, and Opx+Est group, and it is analyzed for the nNOS and actin levels by Western blotting. (B) The relative amount of nNOS protein significantly increased after oophorectomy. It is normalized after Estradiol replacement. *p<0.05. Con, control; Opx, oophorectomy; Opx+Est, 17β-estradiol replacement after oophrorectomy. |
 | FIG. 5Immunoreactivity pattern for endothelial nitric oxide synthase (eNOS) of rat bladder (immunohistochemical stain, ×400). The expression of eNOS increased in Opx group in comparison to the Con group (A), while it decreased in the Opx+Est group (B) in comparison to the Opx group (C). Con, control; Opx, oophorectomy; Opx+Est, 17β-estradiol replacement after oophrorectomy. |
 | FIG. 6Endothelial nitric oxide synthase (eNOS) protein expression in the rat bladder. (A) Protein is extracted from the bladder in the Con, Opx, and Opx+Est group, and it is analyzed for the eNOS and actin levels by Western blotting. (B) The relative amount of eNOS protein significantly increased after oophorectomy. It is normalized after Estradiol replacement. *p<0.05. Con, control; Opx, oophorectomy; Opx+Est, 17β-estradiol replacement after oophrorectomy. |
 | FIG. 7Masson's trichrome staining of the rat bladder (Masson's trichrome staining, ×200). The proliferation of epithelial cells and the increase in the muscle layer of the bladder were observed for the control (Con) group (A), Opx+Est group (B), Opx group (C). Opx, oophorectomy; Opx+Est, 17β-estradiol replacement after oophrorectomy. |
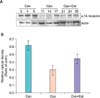 | FIG. 8α1A receptor protein expression in the rat urethra. (A) Protein is extracted from the urethra in the Con, Opx, and Opx+Est group, and it is analyzed for the α1A receptor and actin levels by Western blotting. (B) The relative amount of α1A receptor protein decreased after oophorectomy. It is normalized after Estradiol replacement. However, the expressions of α1A receptor protein in the Con, Opx, and Opx+Est group are not significantly different. Con, control; Opx, oophorectomy; Opx+Est, 17β-estradiol replacement after oophrorectomy. |
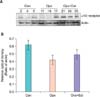 | FIG. 9α1D receptor protein expression in the rat urethra. (A) Protein is extracted from the urethra in the Con, Opx, and Opx+Est group, and it is analyzed for the α1D receptor and actin levels by Western blotting. (B) The relative amount of α1D receptor protein increased after oophorectomy. It is normalized after Estradiol replacement. However, the expressions of α1D receptor protein in the Con, Opx, and Opx+Est group are not significantly different. Con, control; Opx, oophorectomy; Opx+Est, 17β-estradiol replacement after oophrorectomy. |
 | FIG. 10Immunreactivity pattern for endothelial nitric oxide synthase (NOS) of rat urethra (immunohistochemical stain, ×400). The expression of neuronal NOS increased in all of the mucous membrane, smooth muscle and blood vessel of the urethra in the Opx group (B). (A) Con, control; (B) Opx, oophorectomy. (C) Opx+Est, 17β-estradiol replacement after oophrorectomy. |
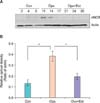 | FIG. 11Neuronal nitric oxide synthase (nNOS) protein expression in the rat urethra. (A) Protein is extracted from the urethra in the Con, Opx, and Opx+Est group, and it is analyzed for the nNOS and actin levels by Western blotting. (B) The relative amount of nNOS protein significantly increased after oophorectomy. It is normalized after Estradiol replacement. *p<0.05. Con, control; Opx, oophorectomy; Opx+Est, 17β-estradiol replacement after oophrorectomy. |
 | FIG. 12Immunoreactivity pattern for endothelial nitric oxide synthase (eNOS) of rat urethra (immunohistochemical stain, ×400). The expression of eNOS increased in Opx group (B) in comparison to the Con group (A), while it decreased in the Opx+Est group (C) in comparison to the Opx group. Con, control; Opx, oophorectomy; Opx+Est, 17β-estradiol replacement after oophrorectomy. |
 | FIG. 13Endothelial nitric oxide synthase (eNOS) protein expression in the rat urethra. (A) Protein is extracted from the urethra in the Con, Opx, and Opx+Est group, and it is analyzed for the eNOS and actin levels by Western blotting. (B) The relative amount of eNOS protein significantly increased after oophorectomy. It is normalized after Estradiol replacement. *p<0.05. Con, control; Opx, oophorectomy; Opx+Est, 17β-estradiol replacement after oophrorectomy. |
 | FIG. 14Masson's trichrome staining of the rat urethra (Masson's trichrome staining, ×200). The increase in the distribution of blood vessels, proliferation of epithelial cells and the increase in the muscle layer of the urethra were observed for the Opx+Est group (C). (A) Con, control; (B) Opx, oophorectomy. (C) Opx+Est, 17β-estradiol replacement after oophrorectomy. |
References
1. Takahashi W, Yoshida M, Wada Y, Goto S, Inadome A, Yono M, et al. Effect of estrogen on nitric oxide-induced relaxation of the rabbit urethra. Eur J Pharmacol. 1997; 339:165–171.
2. Morrison JF. The functions of efferent nerves to the lower urinary tract. In : Torrens M, Morrison JF, editors. The physiology of the lower urinary tract. London: Springer;1987. p. 133–159.
3. Seguchi H, Nishimura J, Zhou Y, Niiro N, Kumazawa J, Kanaide H. Expression of beta3-adrenoceptors in rat detrusor smooth muscle. J Urol. 1998; 159:2197–2201.
4. Maggi CA, Santicioli P, Furio M, Meli A. Dual effects of clonidine on micturition reflex in urethane anesthetized rats. J Pharmacol Exp Ther. 1985; 235:528–536.
5. Hampel C, Dolber PC, Smith MP, Savic SL, Throff JW, Thor KB, et al. Modulation of bladder alpha1-adrenergic receptor subtype expression by bladder outlet obstruction. J Urol. 2002; 167:1513–1521.
6. Dokita S, Morgan WR, Wheeler MA, Yoshida M, Latifpour J, Weiss RM. NG-nitro-L-arginine inhibits non-adrenergic, noncholinergic relaxation in rabbit urethral smooth muscle. Life Sci. 1991; 48:2429–2436.
7. Hashimoto S, Kigoshi S, Muramatsu I. Nitric oxide-dependent and -independent neurogenic relaxation of isolated dog urethra. Eur J Pharmacol. 1993; 231:209–214.
8. Leone AM, Wiklund NP, Hokfelt T, Brundin L, Moncada S. Release of nitric oxide by nerve stimulation in the human urogenital tract. Neuroreport. 1994; 5:733–736.
9. Yallampalli C, Byam-Smith M, Nelson SO, Garfield RE. Steroid hormones modulate the production of nitric oxide and cGMP in the rat uterus. Endocrinology. 1994; 134:1971–1974.
10. Jackson SL, Fihn SD. Exogenous estrogen and urinary incontinence. J Urol. 2009; 181:1989–1991.
11. Chen B, Yeh J. Alterations in connective tissue metabolism in stress incontinence and prolapse. J Urol. 2011; 186:1768–1772.
12. Hextall A, Cardozo L. The role of estrogen supplementation in lower urinary tract dysfunction. Int Urogynecol J Pelvic Floor Dysfunct. 2001; 12:258–261.
13. Grady D, Brown JS, Vittinghoff E, Applegate W, Varner E, Snyder T, et al. Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol. 2001; 97:116–120.
14. Moehrer B, Hextall A, Jackson S. Oestrogens for urinary incontinence in women. Cochrane Database Syst Rev. 2003; (2):CD001405.
15. Beisland HO, Fossberg E, Moer A, Sander S. Urethral sphincteric insufficiency in postmenopausal females: treatment with phenylpropanolamine and estriol separately and in combination. A urodynamic and clinical evaluation. Urol Int. 1984; 39:211–216.
16. Dmitrieva N. Increased alpha1-adrenergic activity in the rat bladder by depletion of ovarian hormones. J Urol. 2007; 178:2677–2682.
17. Rand MJ, Li CG. Discrimination by the NO-trapping agent, carboxy-PTIO, between NO and the nitrergic transmitter but not between NO and EDRF. Br J Pharmacol. 1995; 116:1906–1910.
18. Bennett BC, Kruse MN, Roppolo JR, Flood HD, Fraser M, de Groat WC. Neural control of urethral outlet activity in vivo: role of nitric oxide. J Urol. 1995; 153:2004–2009.
19. Ho MH, Bhatia NN, Khorram O. Physiologic role of nitric oxide and nitric oxide synthase in female lower urinary tract. Curr Opin Obstet Gynecol. 2004; 16:423–429.
20. Okuno T, Masuda H, Tsujii T, Kihara K, Yamauchi Y, Azuma H. Accumulated endogenous nitric oxide synthase inhibitors in inhibiting urethral relaxation following estrogen supplementation in ovariectomized rabbits. J Urol. 2004; 172:360–364.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download