Abstract
Purpose
Antenatal hydronephrosis (AH) is found in 0.5%-1% of neonates. The aim of the study was to assess the urinary concentrations of 3 biomarkers, endothelin-1 (ET-1), monocyte chemotactic peptide-1 (MCP-1), and N-acetyl-glucosaminidase (NAG) in severely hydronephrotic neonates.
Materials and Methods
Neonates with a history of prenatal hydronephrosis were enrolled in the prospective study in 2 groups. Group 1 included neonates with severe forms of obstruction requiring surgical intervention and group 2 included neonates with milder forms of obstruction without any functional impairment. Fresh voided urinary levels of ET-1, MCP-1, and NAG were measured and their ratios to urinary Cr were calculated.
Results
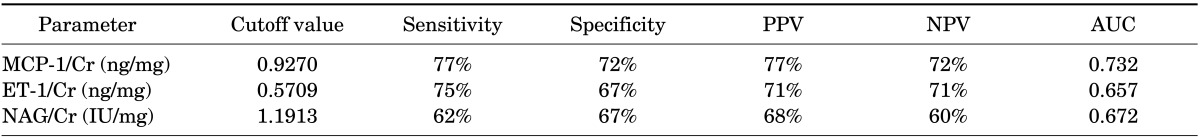
Fourty-two neonates were enrolled into the 2 groups: group 1, 24 patients (21 male, 3 female); group 2, 18 neonates (16 male, 2 female). There were no statistically significant differences between urinary ET-1, NAG, MCP-1 values, and ET-1/Cr and NAG/Cr ratios in groups 1 and 2. The urinary MCP-1/Cr ratio was significantly higher in group 1 than in group 2. For comparison of groups 1 and 2, the cut-off values were measured as 0.5709 ng/mg (sensitivity, 75%; specificity, 67%; positive predictive value [PPV], 71%; negative predictive value [NPV], 71%), 0.927 ng/mg (sensitivity, 77%; specificity, 72%; PPV, 77%; NPV, 72%), and 1.1913 IU/mg (sensitivity, 62%; specificity, 67%; PPV, 68%; NPV, 60%) for ET-1/Cr, MCP-1/Cr, and NAG/Cr ratios, respectively.
Antenatal hydronephrosis (AH) is a relatively common finding on gestational ultrasound: it is observed in about 0.5% to 1% of pregnant women, especially after the 28th gestational week [1,2]. The most common etiologies are unilateral or bilateral obstructive uropathy [3]. Ureteropelvic junction obstruction (UPJO) is the most common finding in fetuses with AH and in neonates with unilateral hydronephrosis. Ureterovesical junction obstruction is the second most common obstructive disorder in these neonates [1,4].
The diagnosis and treatment of neonates with AH is controversial [1,2]. It has been suggested that most babies do not need any treatment other than observation and close follow-up [5]. In infants whose renal function is severely reduced owing to obstructive uropathy, prompt surgical treatment is recommended [6,7]. The current tools for assessment of renal differential function and obstruction are renal scintigraphy modalities, such as 99mTc-MAG3, 99mTc-DTPA, and 99mTc-EC scans [5,6,7]. This technique has some limitations, including cost, exposure to ionizing radiation, invasiveness (because of the need for injections in all cases and catheterization in many cases), and finally inaccessibility in some countries [8]. Therefore, development of a diagnostic modality that is less invasive and has no risk of exposure to toxic chemicals is necessary. Urinary biomarkers are candidates for assessment of kidney injury and have been studied widely for this purpose [9,10]. Theoretically, these markers could be used for the early diagnosis of kidney damage in cases with obstruction [11,12].
In this study, we assessed the urinary levels of 3 markers in neonates: endothelin-1 (ET-1), monocyte chemotactic peptide-1 (MCP-1), and N-acetyl-beta-D-glucosaminidase (NAG). These biomarkers are potentially related to the renal cell injury that occurs before the clinical appearance of the insult. ET-1 is a strong vasoconstrictor that has a role in the development of stenosis in the preglomerular arterioles. It is a mediator of vascular and cellular damage in the course of urinary system obstruction [13,14]. MCP-1 is a peptide with 76 amino acids that belongs to the family of chemokines and exerts strong chemoattractant activities on monocytes, T cells, and natural killer cells. It plays an important role in acute and chronic human tubulointerstitial diseases [15,16]. NAG is a lysosomal enzyme that is abundantly present in the cells of the proximal tubule. It is considered to be a very sensitive marker of renal tubular impairment in various disease states [17,18,19]. Low NAG levels are found in normal urine as a result of the exocytosis/endocytosis transport system of the proximal tubules. Any perturbation of this system results in an elevated excretion of different NAG isoenzymes, including intermediate forms, which then provides an excellent early indicator of any kind of tubular damage [19].
Neonates with a prenatal history of hydronephrosis were enrolled in this study. The prospective study was approved by the Research Committee of Mazandaran University of Medical Sciences (approval number #4/P/674) and was performed from March 2012 to January 2014. All parents provided signed, written informed consent before enrollment of their neonates into this study. AH was defined as an anteroposterior (AP) pelvic diameter of >7 mm after the 36th gestational week and >5 mm before the 36th gestational week on an ultrasound exam. Postnatal ultrasonography was performed on days 3-7 and weeks 6-8 on the basis of our hospital policy. The severity of hydronephrosis was assessed according to the Society of Fetal Urology criteria and is presented as grade 1 to grade 4. Cystography was performed at week 1 for neonates with severe bilateral hydronephrosis and at weeks 6-8 in other infants with significant persistent postnatal hydronephrosis. Those with vesicoureteral reflux (VUR) were excluded from the study.
Neonates without VUR who had persistent significant hydronephrosis (grade 3 or 4, or pelvic diameter >15 mm) were studied by dynamic renal scan by use of 99mTc-MAG3, 99mTc-DTPA, and 99mTc-EC radiopharmaceuticals for assessment of the presence of any obstruction and the severity of renal function impairment. The obstruction was defined as a time to reduction of tracer uptake to half value (T1/2) of more than 15 minutes. A differential function of less than 40% was considered as functional impairment of the renal unit. The indications for surgery were differential renal function <40%, bilateral obstruction, unilateral obstruction in a solitary kidney, and severely symptomatic patients. In this study, patients were categorized into two groups: group 1 included neonates who had severe hydronephrosis and were candidates for surgical intervention on the basis of functional impairment of the involved kidney (differential renal function, <40%); group 2 included neonates with less severe hydronephrosis with preserved renal function who did not need any surgical treatment. Those with VUR, posterior urethral valve, and other postbladder obstructions were excluded from the study.
All neonates were assessed for blood urea nitrogen, plasma creatinine (Cr), and urinary levels of ET-1, MCP-1, NAG, and Cr. Fresh first-morning voided urine samples were obtained in the first week before contrast studies. The samples were collected in sterile polypropylene containers. One-milliliter aliquots were centrifuged at 4000×g for 10 minutes and the supernatant fraction was stored at -80℃ until analysis. The urine was tested for the presence of blood or leucocytes by use of urine analysis strips; samples containing blood or leucocytes were excluded. All samples were analyzed in duplicate and blinded to clinical status.
Urinary levels of ET-1 and MCP-1 were determined by use of commercially available immunoassays (Abcam, Cambridge, UK) according to the manufacturer's protocol. In addition, urinary NAG concentrations were measured by a colorimetric assay according to the manufacturer's recommendation (Boehringer-Mannheim GmbH, Ingelheim am Rhein, Germany). In brief, samples were placed into wells coated with a mouse monoclonal antibody as a catcher. After washing, biotinylated antihuman MCP1, ET-1, or NAG antibody was added. Unbound antibodies washed away, and a horseradish peroxidase-conjugated streptavidin was used for detection of the presence of 3,3',5,5'-Tetramethylbenzidine substrate. Reference concentrations of ET-1, MCP-1, and NAG were used for assay calibration. Absorption was determined with an enzyme-linked immunosorbent assay (ELISA) reader (Biotek ELX800, Bio-Tek Instruments Inc., Winooski, VT, USA) at 450 nm. The assay sensitivities were 1.1 and 0.41 ng/L for MCP-1 and ET-1 and 5 U/L for NAG, respectively. Inter- and intra-assay coefficients of variation were below 10%. For each assay, the results were calculated with reference to a standard curve. The sensitivities of the assays were 1.1 ng/L for MCP-1, 0.41 ng/L for ET-1, and 5 U/L for NAG, respectively. All samples were analyzed in duplicate with the appropriate standards on 96-well microplates. For practical purposes, we collected random urinary measures and then normalized the results to urinary Cr values to modulate the daily changes in urinary excretion of markers. The values were expressed in ng/mg or U/mg Cr.
Categorical variables were presented as percentages, whereas continuous variables were expressed as means±standard deviation or median (25-75th quartiles). The normality of distribution was assessed by using the Kolmogorov-Smirnov test. Statistical analysis of the difference between groups with normal distributions was determined by using the t-test or Fisher exact test to compare two groups. The chi-square test was used for the qualitative data. Nonparametric tests such as the Mann-Whitney test were used for variables that were not distributed normally. A receiver operating characteristic (ROC) curve was drawn and the sensitivity and specificity of the different cutoff points for ET-1, MCP-1, NAG, ET-1/Cr, MCP-1/Cr, and NAG/Cr were determined. The best cutoff point was chosen according to the ROC curve. In addition, the area under the curve was calculated. The results were expressed with a 95% confidence interval (CI). A p-value of <0.05 was considered statistically significant. All statistical analysis was performed by using SPSS ver. 16 (SPSS Inc., Chicago, IL, USA).
Forty-two neonates were enrolled in this study and classified into two groups: group 1 included neonates with AH who were candidates for surgical intervention (n=24, 21 males and 3 females); group 2 included neonates with AH with milder degrees of obstruction who did not need any surgical intervention (n=18, 16 males and 2 females). The demographic and other characteristics of the study population are summarized in Table 1. There were no significant differences in sex, gestational maturity, or type of delivery between the groups (p=0.89, p=0.83, and p=0.66 respectively). Surgery was performed in all group 1 patients at a mean age of 4.24 months for functional impairment. In this study, 27 patients (64%) had unilateral hydronephrosis; however, there was no significant difference in laterality in the two groups of patients (p=0.09). As shown in Table 2, most neonates in group 1 had severe degrees of hydronephrosis in the pre- and postnatal period.
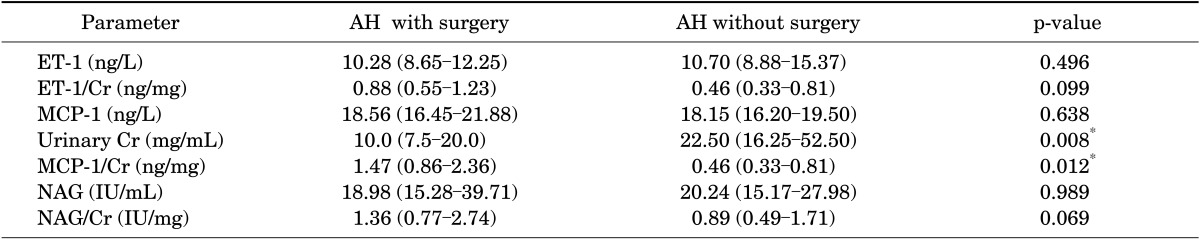
The median urinary levels and 25th and 75th quartiles of the biomarkers (ET-1, MCP-1, and NAG) are presented in Table 3. For standardization purposes, we calculated values as a ratio to urinary Cr (ET-1/Cr, MCP-1/Cr, and NAG/Cr). The Kolmogorov-Smirnov test was used for assessment of normality of distribution; none of the distributions met the criteria for normality, so nonparametric tests were performed. In comparison between the two groups, there were no significant differences for absolute measures of ET-1, MCP-1, and NAG. When we standardized the values to urinary Cr, however, some differences appeared. The ET-1/Cr ratio was not significantly different between the two obstructive groups. The NAG/Cr ratio was higher in group 1 than group 2, but the difference was not statistically significant. The MCP-1/Cr ratio was significantly higher in group 1 than in group 2 (1.47 ng/mg vs. 0.46 ng/mg, respectively; p=0.012).
The sensitivity and specificity of the ET-1/Cr, MCP-1/Cr, and NAG/Cr ratios were calculated from the ROC curves (Fig. 1). Groups 1 and 2 were compared to find the cutoff value for distinguishing neonates with severe obstructive hydronephrosis from those with milder forms without any indication for surgical intervention.
For comparison of groups 1 and 2, the cutoff values were measured as follows: 0.5709 ng/mg (sensitivity, 75%; specificity, 67%; positive predictive value [PPV], 71%; negative predictive value [NPV], 71%) for ET-1/Cr, 0.927 ng/mg (sensitivity, 77%; specificity, 72%; PPV, 77%; NPV, 72%) for MCP-1/Cr, and 1.1913 IU/mg (sensitivity, 62%; specificity, 67%; PPV, 68%; NPV, 60%) for NAG/Cr ratios (Table 4).
Urinary tract obstruction in the perinatal period is a challenge for clinicians. The risk of permanent renal damage warrants surgical intervention in the first year of life [20,21]. Assessment of differential renal function by scintigraphic studies is at the center of decision-making strategies. These radioisotope studies are relatively invasive, however. In this study, we evaluated the role of urinary biomarkers for this purpose.
The cellular effect of urinary obstruction comprises three basic mechanisms: (1) leukocyte (especially macrophage) migration and interstitial infiltration, (2) vasogenic effects and ischemia, and (3) cellular death and apoptosis. The markers for each of the 3 pathways are MCP-1 for the first stage, ET-1 for the vascular pathway, and NAG for the cellular stage [22]. Despite the common characteristics of the 3 markers, each biomarker has a unique mechanism of action. In our study, only the MCP-1/Cr ratio was significantly higher in the severe obstruction group than in the mild obstruction group. The NAG/Cr ratio was not significantly higher in the severe obstruction group, and the ET-1/Cr ratio was similar in the 2 groups. The immunologic and tubulointerstitial changes in the obstructive process begin to develop in the first weeks of life, but other processes such as vascular or enzymatic processes take more time to develop. The participants in our study were neonates aged less than 2 weeks, and thus our results support this timing of events.
Grandaliano et al. [15] studied 24 children affected by congenital UPJO and 15 healthy children. They measured urinary MCP-1 levels by ELISA and evaluated MCP-1 gene expression by in situ hybridization. They found that urinary MCP-1 concentrations were significantly positively correlated with the mean transit time of 99mTc-MAG3 through the cortex of the obstructed kidney.
Stephan et al. [16] performed an animal study on 48 surgically obstructed male juvenile Wistar rats (28 days old). The renal pelvic urinary concentration and renal mRNA expression of MCP-1 were correlated with the grade of obstruction and indicated the degree of subsequent renal damage in hydronephrosis.
The role of MCP-1 was assessed in two recent studies. Bartoli et al. [23] studied 76 patients with UPJO and 30 normal children. Urinary MCP-1 increased significantly in the UPJO group compared with the control group and significantly improved in the operated group. The results supported the role of urinary MCP-1 as a marker of tubulointerstitial damage in human obstructive nephropathy. Taranta-Janusz et al. [24] performed a prospective case-control study in children with hydronephrosis caused by UPJO. Children were categorized into two study groups: the first group comprised 15 surgical cases with severe hydronephrosis due to a unilateral, critical degree of ureteral stenosis and who underwent pyeloplasty; the second group comprised 21 conservative cases with mild, nonobstructive hydronephrosis who did not require any surgery. A third group was a healthy control group. Those authors reported that urinary MCP-1/Cr levels from voided urine before and after surgery and concentrations obtained from the affected pelvis were significantly greater than in the second group and the control group (p<0.05). The difference in the MCP-1/Cr level from voided urine and the concentration in the affected pelvis was not statistically significant.
Madsen et al. [25] reported on 28 children with unilateral UPJO and 13 healthy sex- and age-matched children. The preoperative concentration of urinary MCP-1 was significantly increased compared with the controls. There was a further increase in the cytokine in the peri- and postoperative (1 day and 3 weeks) samples followed by a decline, but the concentrations in the postoperative (3 months and 1 year) samples were not significantly different from the controls.
We found similar results in our study. The MCP-1/Cr ratio was significantly higher in obstructive neonates with surgical indication. A cutoff ratio with 77% sensitivity and 72% specificity was found.
We did not find the same results for NAG or ET-1. This was due to the age of the patients. One or 2 weeks of postnatal life are not long enough for the vascular or enzymatic pathways of cellular damage induced by obstruction to begin [22]. Sharifian et al. [13] determined a cutoff point of ≥50 pg/dL for the urinary ET-1 level for diagnosis of hydronephrosis in children. The sensitivity and specificity of this test were 88.9% and 75.5%, respectively. Knerr et al. [26] assessed gene expression in 20 tissue specimens obtained from UPJO patients. The expression of ET-1 in the obstructed ureteropelvic junction was significantly higher than in control tissue.
Ma et al. [18] analyzed the clinical urinary NAG value in evaluating the degree of damage to hydronephrotic kidneys in children. Urine NAG levels in healthy and hydro nephrotic kidneys were 3.31×(10-3±0.11)×10-3 IU/L and 5.91×(10-3±0.17)×10-3 IU/L, respectively (p=0.05). There was a significant positive correlation between urinary NAG levels from hydronephrotic kidneys and pathologic grades (r=0.769, p<0.05).
Skalova et al. [17] studied 31 children with hydronephrosis diagnosed by use of abdominal ultrasonography either prenatally or postnatally. The urinary NAG/Cr ratio was significantly higher in the patients with hydronephrosis in comparison with the reference data. This finding, of no relation between the urinary NAG/Cr level and the severity of obstruction, is similar to the results of our study.
There are few studies on neonates with AH. Therefore, our hypothesis that the age of the children is the most important factor in the discrepancy in urinary levels of the three biomarkers may be only a simple assumption. Larger studies with a range of ages are needed to confirm this theory.
References
1. Coplen DE. The Society for Fetal Urology consensus statement on the evaluation and management of antenatal hydronephrosis: Nguyen HT, Herndon CDA, Cooper C, et al (Children's Hosp, Boston, MA; Children's Hosp of Alabama, Birmingham; Univ of Iowa Med Ctr, Iowa City; et al) J Pediatr Urol 6:212-231, 2010. Yearb Urol. 2010; 2010:209.
2. Yamacake KG, Nguyen HT. Current management of antenatal hydronephrosis. Pediatr Nephrol. 2013; 28:237–243. PMID: 22836304.

3. Mohammadjafari H, Alam A, Kosarian M, Mousavi SA, Kosarian S. Vesicoureteral reflux in neonates with hydronephrosis; role of imaging tools. Iran J Pediatr. 2009; 19:347–353.
4. Vazirian S, Mohammadjafari H, Mohammadjafari R. Postnatal management of prenatal hydronephrosis. J Clin Excell. 2013; 1:63–82.
5. Ucero AC, Benito-Martin A, Izquierdo MC, Sanchez-Nino MD, Sanz AB, Ramos AM, et al. Unilateral ureteral obstruction: beyond obstruction. Int Urol Nephrol. 2014; 46:765–776. PMID: 24072452.

6. Cao YS, Yu DX, Cai Y, Chao M. Diagnosis and treatment of ureteropelvic junction obstruction in children. J Appl Clin Pediatr. 2007; 22:825.
7. Klein J, Gonzalez J, Miravete M, Caubet C, Chaaya R, Decramer S, et al. Congenital ureteropelvic junction obstruction: human disease and animal models. Int J Exp Pathol. 2011; 92:168–192. PMID: 20681980.

8. Abedi SM, Mohammadjafari H, Hosseinimehr SJ, Mardanshahi A, Shahhosseini R. Imaging of renal cortex in nuclear medicine. J Clin Excell. 2014; 2:50–69.
9. Mohammadjafari H, Rafiei A, Abedi M, Aalaee A, Mirabi AM, Abedi E. Urinary neutrophil-gelatinase associated lipocalin is a more prognostic biomarker to distinguish antenatal hydronephrosis in neonates. Res Mol Med. 2013; 1:10–16.

10. Trnka P, Hiatt MJ, Tarantal AF, Matsell DG. Congenital urinary tract obstruction: defining markers of developmental kidney injury. Pediatr Res. 2012; 72:446–454. PMID: 22902433.

11. Chevalier RL. Obstructive nephropathy: towards biomarker discovery and gene therapy. Nat Clin Pract Nephrol. 2006; 2:157–168. PMID: 16932414.

12. Mohammadjafari H, Rafiei A, Abedi M, Aalaee A, Abedi E. The role of urinary TIMP1 and MMP9 levels in predicting vesicoureteral reflux in neonates with antenatal hydronephrosis. Pediatr Nephrol. 2014; 29:871–878. PMID: 24389602.

13. Sharifian M, Ahmadi M, Karimi A, Zand RE, Moghadar R, Ahmadi R, et al. Urinary endothellin-1 level in children with pyelonephritis and hydronephrosis. Saudi J Kidney Dis Transpl. 2013; 24:731–736. PMID: 23816722.

14. Pollock D. Endothelin and the kidney. In : Battistini B, Warner TD, editors. Endothelin and its inhibitors. New York: Springer;2001. p. 477–501.
15. Grandaliano G, Gesualdo L, Bartoli F, Ranieri E, Monno R, Leggio A, et al. MCP-1 and EGF renal expression and urine excretion in human congenital obstructive nephropathy. Kidney Int. 2000; 58:182–192. PMID: 10886563.

16. Stephan M, Conrad S, Eggert T, Heuer R, Fernandez S, Huland H. Urinary concentration and tissue messenger RNA expression of monocyte chemoattractant protein-1 as an indicator of the degree of hydronephrotic atrophy in partial ureteral obstruction. J Urol. 2002; 167:1497–1502. PMID: 11832777.

17. Skalova S, Rejtar P, Kutilek S. Increased urinary N-acetyl-beta-D-glucosaminidase activity in children with hydronephrosis. Int Braz J Urol. 2007; 33:80–83. PMID: 17335604.

18. Ma H, Fang Y, Tian WC, Qian K, Li J, Yang JJ, et al. Clinical value of renal dynamic imaging and urinary N-acetyl-β-D-glucosaminidase, apoptosis DNA fragment detection in evaluating damage degree of hydronephrotic kidneys in children with hydronephrosis. J Appl Clin Pediatr. 2009; 16:036.
19. Csathy L, Pocsi I. Urinary N-acetyl-beta-D-glucosaminidase determination in newborns and children: methods and diagnostic applications. Eur J Clin Chem Clin Biochem. 1995; 33:575–587. PMID: 8611667.
20. Chertin B, Pollack A, Koulikov D, Rabinowitz R, Hain D, Hadas-Halpren I, et al. Conservative treatment of ureteropelvic junction obstruction in children with antenatal diagnosis of hydronephrosis: lessons learned after 16 years of follow-up. Eur Urol. 2006; 49:734–738. PMID: 16504374.

21. Tombesi MM, Alconcher LF. Short-term outcome of mild isolated antenatal hydronephrosis conservatively managed. J Pediatr Urol. 2012; 8:129–133. PMID: 21798811.

22. Chevalier RL, Thornhill BA, Forbes MS, Kiley SC. Mechanisms of renal injury and progression of renal disease in congenital obstructive nephropathy. Pediatr Nephrol. 2010; 25:687–697. PMID: 19844747.

23. Bartoli F, Penza R, Aceto G, Niglio F, D'ddato O, Pastore V, et al. Urinary epidermal growth factor, monocyte chemotactic protein-1, and β2-microglobulin in children with ureteropelvic junction obstruction. J Pediatr Surg. 2011; 46:530–536. PMID: 21376205.

24. Taranta-Janusz K, Wasilewska A, Debek W, Waszkiewicz-Stojda M. Urinary cytokine profiles in unilateral congenital hydronephrosis. Pediatr Nephrol. 2012; 27:2107–2113. PMID: 22744767.

25. Madsen MG, Norregaard R, Palmfeldt J, Olsen LH, Frokiar J, Jorgensen TM. Epidermal growth factor and monocyte chemotactic peptide-1: potential biomarkers of urinary tract obstruction in children with hydronephrosis. J Pediatr Urol. 2013; 9(6 Pt A):838–845. PMID: 23228281.

26. Knerr I, Nyul Z, Miller J, Rosch W, Dotsch J, Repp R, et al. Increased endothelin-1 and decreased adrenomedullin gene expression in the stenotic tissue of congenital pelvi-ureteric junction obstruction in children. BJU Int. 2001; 87:667–671. PMID: 11350409.

FIG. 1
Receiver operating characteristic (ROC) curve to detect severe obstruction (differentiate group 1 from group 2). (A) For endothelin-1 to creatinine ratio (ET-1/Cr), area under the curve (AUC)=0.657 (standard error [SE], 0.094; 95% confidence interval [CI], 0.473-0.841). (B) For monocyte chemotactic peptide-1 to creatinine ratio (MCP-1/Cr) ratio, AUC=0.732 (SE, 0.084; 95% CI, 0.568-0.896). (C) For N-acetyl-beta-D-glucosaminidase to creatinine ratio (NAG/Cr), AUC=0.627 (SE, 0.088; 95% CI, 0.500-0.844).
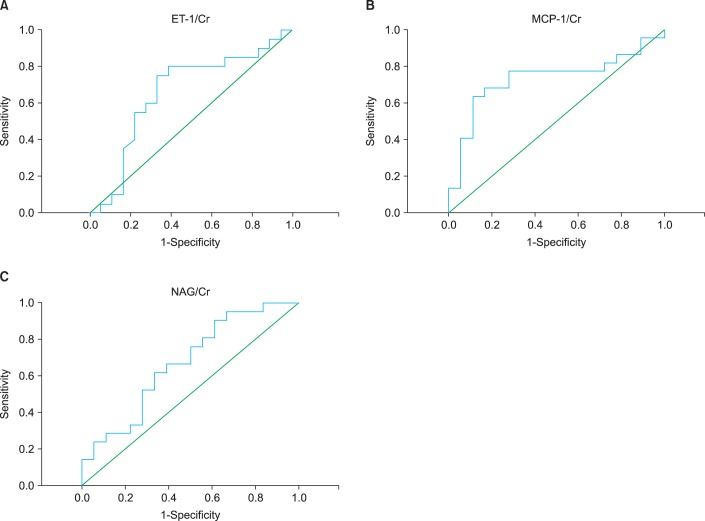
TABLE 1
Demographic and clinical findings of patients with antenatal hydronephrosis with or without obstruction
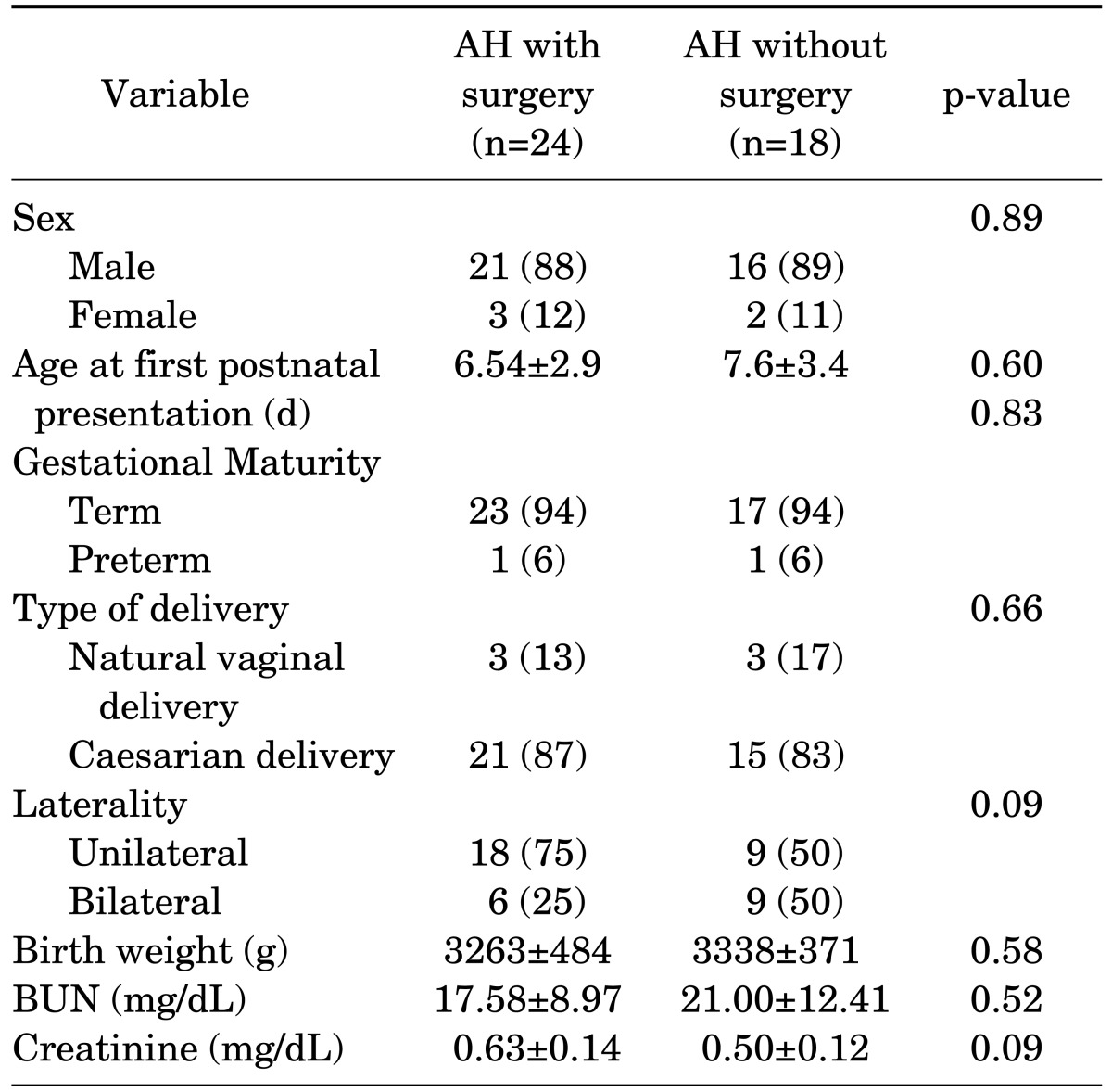
TABLE 2
Ultrasonographic findings in hydronephrotic neonates with and without surgery according to SFU classification
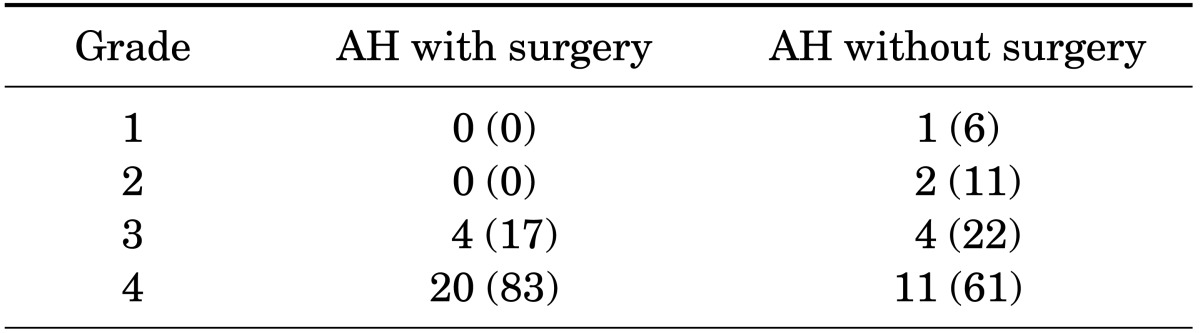
TABLE 3
Urinary ET-1, MCP-1, and NAG levels and their ratio to urinary Cr in hydronephrotic patients with or without obstruction or surgery




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download