Abstract
Purpose
Ultrasound (US) is highly sensitive in the detection of renal masses. However, it may not be able to differentiate benign and malignant lesions in smaller masses. The purpose of this study was to determine the diagnostic efficacy of contrast-enhanced ultrasound (CEUS) for small renal masses.
Materials and Methods
From January 2011 to December 2013, a total of 85 patients underwent CEUS for evaluation of renal masses. Of these patients, CEUS findings were retrospectively analyzed for small renal cell carcinoma (RCC) cases (n=38) and angiomyolipoma (AML) cases (n=11). The tumor echogenicity and enhancement patterns and degrees were evaluated. The diagnostic efficacy of CEUS in differentiating the two diseases was compared.
Results
On CEUS, the findings of diffuse heterogeneous enhancement (observed in 78.9% of RCCs and 27.3% of AMLs, p=0.003), washout from hyperenhancement or iso-enhancement to hypoenhancement in late phase (73.7% of RCCs and 18.2% of AMLs, p=0.001), and perilesional rim-like enhancement (57.9% of RCCs and 9.1% of AMLs, p=0.006) were significantly different between AML and RCC cases. The corresponding sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 86.8% (33/38), 63.6% (7/11), 89.2% (33/37), 58.3% (7/12), and 81.6% (40/49), respectively.
Ultrasound (US) is a reliable imaging technique for the early diagnosis of renal cell carcinoma (RCC). It is a readily accessible, cost-effective, noninvasive imaging modality that provides real-time features. However, it may not provide differentiation in special cases of RCC and renal angiomyolipoma (AML). Although most RCCs are hypoechoic on US, approximately 30% of small RCCs appear hyperechoic, similar to small AMLs, which typically appear very bright, homogeneous, and sharply marginated [1]. In addition, the incidence of atypical iso-hypoechoic and slightly hyperechoic AMLs account for 6% and 29% of small renal masses, respectively. The usefulness of US is limited because of its lower accuracy in the characterization of such small renal masses. RCC is a malignant neoplasm, which requires total or partial nephrectomy, and thus definite distinction between RCC and AML is essential. Patients with uncharacterized small renal masses have to be examined by further imaging study such as contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI). However, the sensitivity and specificity for the differentiation of RCC from other tumors has been reported to be from 73% to 100% and from 84% to 91%, respectively. Additionally, issues of nephrotoxicity of the contrast media, radiation hazard, and high cost have also been raised [2].
Recently, the development of new contrast media and imaging techniques has enabled contrast-enhanced ultrasound (CEUS), which is already being actively used in organs such as the liver. CEUS can be used to observe the continuous micro- and macrocirculation of a renal mass, which may also be useful in the diagnosis of a renal mass, although the blood supply of renal masses and liver masses differs. In addition, the ultrasound contrast agent is relatively harmless with a lower incidence of side effects such as nephrotoxicity [3]. The purpose of this study was to determine whether CEUS could improve the diagnostic accuracy of US examination for small renal masses.
Between January 2011 and December 2013, a total of 85 consecutive patients underwent CEUS after being diagnosed with renal masses first detected by baseline US. The CEUS studies were performed by a single radiologist (Y.H.L.) at our institution (Wonkwang University School of Medicine & Hospital). Among the patients, 49 with solid renal tumors with diameters of 4 cm or less were included in this study. The 49 included patients were 35 men (71.4%) and 14 women (28.6%) aged 37 to 83 years (mean age, 61 years). The remaining 36 patients were excluded because of complicated renal cysts. Specimens were obtained from surgical resections, which were all performed by a single surgeon (I.Y.S.). The final diagnosis was confirmed by histopathological examination as RCC (n=38) or AML (n=11). After obtaining approval from the Institutional Review Board, we retrospectively reviewed the imaging findings and clinical records.
The US contrast agent used in our study was SonoVue (Bracco, Milan, Italy), a sulfur hexafluoride-filled microbubble contrast agent that was stabilized by phospholipids. A bolus of the ultrasound contrast agent was administered into the antecubital vein [4]. Perfusion through the renal mass was checked in real time for 3 to 4 minutes from the beginning of injection. Injections were repeated 15 minutes after the first injection if a tumor was incompletely assessed owing to its large size [5]. Conventional US was used to scan the renal mass, and the tumor was targeted. Then, color Doppler sonography was performed to estimate the vascularity of the renal mass such as pulsatile arterial or continuous venous flow. Afterward, CEUS was initiated. The tumor and renal cortex were observed for at least 3 minutes. A timer was started immediately after the injection of the ultrasound contrast agent. Digital video clips of characteristic conventional US and CEUS images were stored for off-line analysis.
A routine diagnostic approach, consisting of conventional ultrasound and CEUS, was applied in each case. On conventional US, the lesion echogenicity was divided into hypoechoic and hyperechoic regions with respect to the echogenicity of the renal cortex. CEUS images of characteristic changes during enhancement were evaluated and recorded, e.g., the tumor enhancement extent, pattern, and their dynamic change along with the CEUS process, as well as other special changes during enhancement. The enhancement pattern of the tumor in comparison with the normal renal cortex was classified as hyperenhancement, hypoenhancement, and nonenhancement [6]. Perilesional rim-like enhancement was defined as a peripheral nodular lesion with centripetal fill-in enhancement around the tumor, which became more distinct in the late phase (Fig. 1) [7]. The conventional US appearance of renal AML is that of a typically hyperechoic lesion against the backdrop of a hypoechoic renal cortex, owing to the other components in the interfaces between fatty and nonfatty tissue of the mass. In CEUS, AML typically appears as a hypoenhancing lesion with a progressive hypoenhancement in the late phase (Fig. 2). In a recent study, AML of the liver appeared as a dot-like pattern with a wide range of enhancement patterns (fragmentation) without sharp discrimination, in comparison with hepatocellular carcinoma [8]. After the image analysis, characteristic enhancement features of RCC and AML were selected. The value of these features was calculated, independently and in various combinations, in differentiating the diagnosis between RCC and AML. On the basis of the characteristic images, the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for RCC diagnostic efficacy were calculated.
All statistical analyses were performed by using IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). Averaged data are presented as mean±standard deviation (SD). The results in the differences of the enhancement time between RCC and AML were compared by using the Mann-Whitney test. Fisher exact test was applied to compare the differences in the characteristic image of RCC and AML on CEUS. The results were considered significant at a p-value of <0.05.
The diameter of the renal masses ranged from 1.2 to 4.0 cm (mean, 2. 89±0.81 cm) for RCCs and from 1.3 to 4.0 cm (mean, 2.85±0.85 cm) for AMLs. The size of the renal masses was not significantly different between RCCs and AMLs (p=0.904). On CEUS, the intervals between the beginning of contrast injection and initial enhancement of the renal cortex for 38 RCCs were 15.3±5.2 seconds (range, 8-24 seconds) and 17.5±5.9 seconds (range, 8-29 seconds), respectively (p>0.05). The corresponding intervals for the 11 AMLs were 14.8±5.5 seconds (range, 9-25 seconds) and 14.1±5.6 seconds (range, 8-22 seconds; p>0.05). Also, there was no significant difference between RCCs and AMLs in the timing of enhancement (p=0.631).
On conventional US, RCCs were heterogeneously or homogeneously hypoechoic in 23 cases (23/38, 60.5%) and hyperechoic in 15 cases (15/38, 39.5%). For AMLs, 3 cases (3/11, 27.3%) were hypoechoic, whereas 8 cases (8/11, 72.7%) were hyperechoic (Fig. 2). The echogenicity of renal tumors observed in conventional US was similar to the findings of other studies, but approximately 39.5% of small RCCs were hyperechoic in our study (Table 1).
The enhancement degree and pattern were different between the two types of renal masses (Table 2). The RCCs usually showed heterogeneous enhancement (30/38, 78.9%) rather than homogeneous enhancement (8/38, 21.1%), whereas most AMLs showed homogeneous enhancement (8/11, 72.7%) rather than heterogeneous enhancement (3/11, 27.3%). Sustained hyperenhancement was observed in most (9/11, 81.8%) of the AMLs, whereas washout from hyperenhancement to hypoenhancement was observed in most (28/38, 73.7%) of the RCCs. In 23 of 49 tumors, rim-like enhancement around the tumors was found in various vascular phases. During the cortical phase, perilesional rims were hyperenhancing or iso-enhancing, whereas in the corticomedullary and late phases, the rims all became hyperenhancing relative to the adjacent renal cortex (Fig. 1). These characteristic images were able to show the boundary of the tumor more distinctly and may be a criterion for partial nephrectomy.
Twenty-two of 38 RCCs (57.9%) showed perilesional rim-like enhancement, whereas 1 of 11 AMLs (9.1%) showed incomplete perilesional rim-like enhancement. Most AMLs were observed to have hyperechogenicity on conventional ultrasound and hypoenhancement on CEUS. In the present study, 27.3% of AMLs showed hypoechogenicity, and some atypical AMLs (4/11, 36.4%) showed a rapid hyperenhancement in CEUS. The dot-like pattern (Fig. 2) was observed in 7 of the AML lesions (63.6%) and 3 of the RCC lesions (7.9%). These features were significantly different between the two groups (p=0.000).
Compared with the difference in CEUS findings between clear cell carcinoma and papillary carcinoma, diffuse heterogeneous enhancement was observed in 26 of 33 clear cell carcinomas (78.8%) and in 4 of 5 papillary carcinomas (80.0%). Washout in the late phase was observed in 24 of 33 clear cell carcinomas (72.7%) and in 4 of 5 papillary carcinomas (80.0%). Perilesional rim-like enhancement was observed in 19 of 33 clear cell carcinomas (57.6%) and in 3 of 5 papillary carcinomas (60.0%). Statistical analysis using a small number of papillary carcinoma patients was difficult, but both groups showed similar characteristics in the CEUS findings (Table 3).
The features of diffuse heterogeneous enhancement, washout, and perilesional rim-like enhancement differed significantly between RCCs and AMLs (p<0.05). Using these features, CEUS had the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for RCC diagnostic efficacy of 86.8% (33/38), 63.6% (7/11), 89.2% (33/37), 58.3% (7/12), and 81.6% (40/49), respectively (Table 4).
Since its introduction into clinical practice, the usefulness of conventional US for earlier diagnosis of renal masses has been well established. In recent studies, as many as 70% of renal tumors were reported to have been discovered incidentally [9,10], and almost 83% of these asymptomatic tumors were originally found in a baseline US examination [11]. Although US has been widely used for evaluating the kidney, many reports have shown its limitations for tumor detection and characterization. About 77% of small RCCs were observed to have various echogenicity, and a minority showed hyperechogenicity (32%) such that these lesions could not be distinguished from renal AMLs [1]. Convertsely, atypical AMLs containing a small amount of fat or less vascular structure often appear as iso- or hypoechoic on conventional US [12]. Therefore, conventional US has limited ability to characterize renal tumors, and further imaging examination such as CT or MRI is currently required.
Already, CEUS has been shown to have high diagnostic efficacy in characteristic images of liver tumors compared with conventional US [13]. Unlike the contrast agents used in CT and MRI, CEUS contrast agents are present in microbubbles, which do not diffuse through the vascular endothelium into the interstitium [14]. These molecular features allow evaluation of both the micro- and macrocirculation of kidney and tumor tissues and can provide a better approach of vascular morphology and characteristic image enhancement. In addition, second-generation US contrast agents have been reported to increase the diagnostic confidence for renal tumors in terms of improved renal lesion conspicuity and effective delineation of tumor microvessels [15]. Most importantly, there are no reported side effects such as nephrotoxicity in humans to date [3]. Also, the lack of renal excretion explains the absence of renal toxicity of these agents, which can, therefore, be used in nephropathic patients.
In the present study, diffuse heterogeneous enhancement, washout in the late phase, and perilesional rim-like enhancement were the most common findings for RCCs. These were characteristic patterns in RCCs with sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 86.8%, 63.6%, 89.2%, 58.3%, and 81.6%, respectively.
Hemorrhage, necrosis, and cystic change are frequently observed pathologic features of RCC. It is generally acknowledged that homogeneity in contrast-enhanced dynamic CT correlates with the existence of necrotic degeneration or intratumoral cysts in histologic specimens [16]. Also, the washout and heterogeneous enhancement pattern on CEUS might relate to the pathologic features of RCC such as a large amount of intratumoral blood flow, numerous thin-walled blood vessels, and hemorrhage. The pseudocapsule results from tumor growth producing compression, ischemia, and necrosis of adjacent normal tissue, which is a subsequent change of fibrous tissue [17]. A recent study reported that perilesional rim enhancement was a characteristic feature of RCC with pathological pseudocapsules on CEUS [7], and the authors of that study concluded that CEUS could be used for ultrasound diagnosis of pseudocapsules in RCC and that the sensitivity of this technique may be competitive to that of MRI in presurgical detection of pseudocapsules. In the present study, perilesional rim-like enhancement was significantly different between RCC and AML. The presence of pseudocapsules was a useful sign both in the differential diagnosis of RCC and in the choice for nephron-sparing surgery.
In the study by Zhou et al. [8], CEUS was found to possibly improve the diagnostic confidence of RCC, providing abundant information on tumor vasculature and the blood supply. It was expected that the enhancement observed in AML would help to differentiate AML from RCC, because of higher angiogenesis in malignant tumors. Most solid RCCs show lower echogenicity than AML in conventional ultrasound. In the present study, some atypical AMLs showed a rapid enhancement during the arterial phase, which was not significantly different from what was observed in RCC. Hyperechogenicity on conventional US and hypoenhancement on CEUS should not be used as a definitive finding for AML. Also, the relative amounts of fat, smooth muscle, and blood vessels vary, and a subset of AMLs contain a minimal fat component that is only microscopically detectable, which accounted for the preoperative misdiagnosis of RCC in a recent study [18]. In the present study, some AMLs showed an enhancement pattern similar to that of RCCs with hyperenhancement, whereas most AMLs revealed a dot-like pattern on CEUS. The dot-like pattern was a valuable finding for diagnosing AML. These features allow confirmation of renal tumors suspected on conventional ultrasound imaging and differentiation between AML and RCC.
Our study had several limitations. First, only a small number of patients with renal masses were included in this study. Second, CEUS is limited by interference from bowel gas, the ribs, or obesity and can be influenced by lesion locations, as with conventional US. The examination involves an operator-dependent imaging modality and requires sufficient experience, skill, and training. Also, the diagnostic efficacy of CEUS is still lower than that of CT or MRI. A large-scale and prospective study is necessary to further assess and extend these results. Additional techniques, such as quantitative CEUS analysis and other specific images, should be evaluated in future studies.
Our results suggest that CEUS features of diffuse heterogeneous enhancement, washout in the late phase, and perilesional rim-like enhancement allow confirmation of RCC, distinguishing RCC from AML, in small renal masses. CEUS improves the diagnostic efficacy of conventional US. It may decrease the necessity of additional diagnostic studies and support decisions about treatment strategy in patients with small renal masses.
Figures and Tables
FIG. 1
A 42-year-old man with clear cell renal carcinoma. (A) Conventional ultrasonography demonstrated that a hypoechogenic mass with a diameter of 2.8 cm was located in the left lower kidney. (B) Contrast-enhanced ultrasound (CEUS) imaging of the arterial phase showed a diffuse, heterogeneous enhanced tumor. (C) CEUS imaging at the late phase showed washout of the tumor. (D) CEUS imaging at the late phase showed perilesional enhancement with slow centripetal fill in a rim-like pattern.
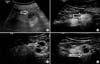
FIG. 2
A 56-year-old man with angiomyolipoma. (A) Conventional ultrasonography demonstrated that a hyperechogenic mass with a diameter of 2.3 cm was located in the left lower kidney. (B) Contrast-enhanced ultrasound imaging in the late phase showed a dot-like enhancement.
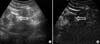
TABLE 1
Comparison of echogenicity and enhancement in patients with RCC or AML according to conventional US and CEUS

ACKNOWLEDGMENTS
This work was an academic award paper at the 2014 Korean Endourological Society Conference.
References
1. Forman HP, Middleton WD, Melson GL, McClennan BL. Hyperechoic renal cell carcinomas: increase in detection at US. Radiology. 1993; 188:431–434.
2. Heidenreich A, Ravery V. European Society of Oncological Urology. Preoperative imaging in renal cell cancer. World J Urol. 2004; 22:307–315.
3. Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008; 29:28–44.
4. Xu ZF, Xu HX, Xie XY, Liu GJ, Zheng YL, Liang JY, et al. Renal cell carcinoma: real-time contrast-enhanced ultrasound findings. Abdom Imaging. 2010; 35:750–756.
5. Lu Q, Wang W, Huang B, Li C, Li C. Minimal fat renal angiomyolipoma: the initial study with contrast-enhanced ultrasonography. Ultrasound Med Biol. 2012; 38:1896–1901.
6. Nilsson A. Contrast-enhanced ultrasound of the kidneys. Eur Radiol. 2004; 14:Suppl 8. P104–P109.
7. Ascenti G, Gaeta M, Magno C, Mazziotti S, Blandino A, Melloni D, et al. Contrast-enhanced second-harmonic sonography in the detection of pseudocapsule in renal cell carcinoma. AJR Am J Roentgenol. 2004; 182:1525–1530.
8. Zhou X, Yan F, Luo Y, Peng YL, Parajuly SS, Wen XR, et al. Characterization and diagnostic confidence of contrast-enhanced ultrasound for solid renal tumors. Ultrasound Med Biol. 2011; 37:845–853.
9. Zhang J, Lefkowitz RA, Ishill NM, Wang L, Moskowitz CS, Russo P, et al. Solid renal cortical tumors: differentiation with CT. Radiology. 2007; 244:494–504.
10. Russo P. Renal cell carcinoma: presentation, staging, and surgical treatment. Semin Oncol. 2000; 27:160–176.
11. Pavlica P, Derchi L, Martorana G, Brunocilla E, Bertaccini A, Manferrari F, et al. Renal cell carcinoma imaging. Eur Urol Suppl. 2006; 5:580–592.
12. Jinzaki M, Tanimoto A, Narimatsu Y, Ohkuma K, Kurata T, Shinmoto H, et al. Angiomyolipoma: imaging findings in lesions with minimal fat. Radiology. 1997; 205:497–502.
13. Quaia E, Palumbo A, Rossi S, Degobbis F, Cernic S, Tona G, et al. Comparison of visual and quantitative analysis for characterization of insonated liver tumors after microbubble contrast injection. AJR Am J Roentgenol. 2006; 186:1560–1570.
14. Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, et al. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004; 232:420–430.
15. Xu ZF, Xu HX, Xie XY, Liu GJ, Zheng YL, Lu MD. Renal cell carcinoma and renal angiomyolipoma: differential diagnosis with real-time contrast-enhanced ultrasonography. J Ultrasound Med. 2010; 29:709–717.
16. Jinzaki M, Tanimoto A, Mukai M, Ikeda E, Kobayashi S, Yuasa Y, et al. Double-phase helical CT of small renal parenchymal neoplasms: correlation with pathologic findings and tumor angiogenesis. J Comput Assist Tomogr. 2000; 24:835–842.
17. Pickhardt PJ, Lonergan GJ, Davis CJ Jr, Kashitani N, Wagner BJ. From the archives of the AFIP. Infiltrative renal lesions: radiologic-pathologic correlation. Armed Forces Institute of Pathology. Radiographics. 2000; 20:215–243.
18. Setola SV, Catalano O, Sandomenico F, Siani A. Contrast-enhanced sonography of the kidney. Abdom Imaging. 2007; 32:21–28.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download