INTRODUCTION
In the last 20 years partial nephrectomy (PN) has emerged as a critical operation for the treatment of renal cortical tumors. Once reserved only for the essential indications of tumors in an anatomically or functionally solitary kidney, today PN can effectively achieve the same local tumor control as radical nephrectomy (RN) while maximally preserving renal function and preventing or worsening pre-existing chronic kidney disease (CKD). The realization that approximately 70% of renal tumors today are detected incidentally with a median size of less than 4 cm and ultimately 45% will have indolent or benign final pathology has further expanded the utilization of PN. The historical use of RN for all renal tumors regardless of size must now be considered obsolete. We have replaced the large and painful eleventh rib flank incision with a mini-flank surgical incision which, when coupled with our rapid recovery clinical pathway, is highly effective for PN and leads to an average 2-day hospital length of stay. This approach is an excellent alternative to the expensive and technically complex minimally invasive approaches (laparoscopic and robotic assisted laparoscopic). It is the purpose of this manuscript to describe in detail our clinical approach to the patient eligible for PN, the intraoperative surgical details, and the management of perioperative complications.
TRANSITION FROM RADICAL TO PARTIAL NEPHRECTOMY
Radical surgical resection of all tumors in the tradition of Halstead was the uniform oncological opinion for the first 70 years of the 20th century. This approach transitioned to organ (i.e., breast) and limb (i.e., sarcoma) sparing operations that provided the same outcomes as their more radical counterparts without severe disability. RN for large, symptomatic, and often metastatic kidney tumors was popularized in the 1960's and is still utilized today in approximately 30% of kidney tumor patients [1,2,3,4,5,6,7,8,9,10,11]. PN was rarely performed and only in patients with tumors in an anatomic or functionally solitary kidney or for those with bilateral synchronous tumors. Advances in complex stone surgery (anatrophic kidney splits, hilar clamping, ice slush, collecting system repair), trauma surgery (renorrhaphy, proximal renal hilar control, vascular repair), and kidney donor transplantation (renoprotective solutions) set the stage for the development of controlled open PN [12,13,14,15,16,17]. The rapid development of the modern imaging modalities of ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI), often ordered for nonspecific abdominal or musculoskeletal complaints, lead to the detection of the small, asymptomatic renal mass [18], which today accounts for 70% of all newly diagnosed renal tumors with a median tumor size of <4 cm (T1a). Approximately 20% of these tumors are benign neoplasms (i.e., oncocytoma, fat poor angiomyolipoma), 25% are indolent malignancies with limited metastatic potential (i.e., papillary type 1, chromophobe) and 54% are the malignant clear cell carcinoma but at the T1 size (<7 cm), metastasis would be realized in less than 10% of patients [18], factors which taken together lead to the expansion of elective PN in the early 1990's [19].
Initially, elective PN was restricted to tumors of 4 cm or less and reports indicated excellent rates of local tumor control and disease-free survival rates of greater than 90% [19,20,21]. Intraoperative US allowed surgeons to approach more complex and often impalpable endophytic, renal sinus, multifocal, and renal hilar tumors with PN [22]. Reports from the United States and abroad indicated oncological equivalency of PN to RN for tumors of 4 cm or less [23,24,25,26]. Expansion of PN for larger (4-7 cm) or higher stage tumors (T2, T3a) located in amenable positions also did not appear to compromise oncological outcomes [27,28,29,30,31,32,33].
NEW CONCERNS REGARDING RENAL FUNCTIONAL PRESERVATION
Based largely on the renal donor transplant experience, it was thought that RN, even for small kidney tumors, would not compromise the patient's long term renal function [34]. However, unlike the carefully selected, young, and healthy kidney donors, kidney tumor patients were on average 25 years older and many suffered from common medical diseases that can affect baseline kidney function such as diabetes, hypertension, and vasculopathy [35,36]. The aging process itself is associated with glomerular atrophy and reduced glomerular filtration rates (GFRs) [37]. Reports appeared describing an elevated postoperative serum creatinine and proteinuria in RN patients compared to PN patients [38,39] and raised concerns that some RN patients were iatrogenically developing a new clinical entity, CKD, described as a GFR of less than 60 mL/min/1.73 m2. CKD affects over 20 million Americans [40,41] and is associated with higher rates of hospitalization, adverse cardiovascular events, and worse overall survival [42]. Using formulas to estimate GFR (eGFR) [43], it was reported that 26% of patients prior to kidney surgery had pre-existing CKD despite a serum creatinine within normal limits and that RN was associated with the causation of CKD or worsening of pre-existing CKD [44]. RN induced CKD also increased the likelihood of adverse cardiovascular events and worse overall survival [45,46,47,48].
Despite the encouragement for PN for small renal masses by the American Urologic Association (AUA) Renal Mass Guideline Committee [49], RN remains over-utilized in the United States and abroad [50,51], particularly in elderly patients and women [52]. This underutilization is likely due to a combination of factors including the introduction of minimally invasive RN, a lack of training in open kidney surgery, and a lack of appreciation for the deleterious renal functional impact of RN. Today, Guidelines Committees recommend PN for all renal tumors <7 cm when technically feasible [49,53].
PARTIAL NEPHRECTOMY: INDICATIONS, PREOPERATIVE ASSESSMENT AND SURGICAL PLANNING
An absolute indication for PN is considered in patients with a tumor in a functional or anatomical solitary kidney. A relative indication for PN is in patients with underlying medical conditions such that RN of the tumor bearing kidney would cause severe renal insufficiency. Elective PN is in patients undergoing resection of kidney tumor in the presence of a healthy contralateral kidney. Preoperative assessment begins with a careful history with a special emphasis on medical comorbidities affecting the cardiovascular and renal function, including cigarette smoking, hypertension, diabetes, and coronary artery disease, and a thorough physical examination. Correctable and potentially life threatening cardiovascular disease should be addressed (i.e., carotid endarectomy, coronary revascularization) prior to PN. A baseline calculation of eGFR should be done using the following web link for the modification of diet in renal disease or CKD-epi equation (http://www.nephron.com/MDRD_GFR.cgi). Preoperative renal protocol CT imaging must have non contrast views in search of microscopic fat or pseudo enhancement with volume averaging to exclude benign angiomyolipoma and hemorrhagic cyst which could warrant nonoperative management [54]. A contrast enhanced study (MRI, CT or renal perfusion/excretion nuclear scan) is required to document bilateral renal function. Renal US with Doppler imaging is an effective way to assess the lesion for vascular flow [55], can characterize cystic lesions, and serve as a template for intraoperative US in order to locate small, impalpable subcortical renal tumors [22].
Although controversy surrounds the routine use of preoperative renal biopsy for renal tumor patients because of concerns for a high rate of nondiagnostic tests and low specificity [56], improved technique and specimen evaluation, have led to improved accuracy and enhanced ability to diagnose tumor histological sub type [57]. We selectively consider tumor biopsy when this information may dramatically change management if, for example, a renal lymphoma is considered. We do not biopsy tumors of elderly, comorbidly ill or frail individuals with small renal tumors and limited life expectancy since the information does not change nonoperative management plans.
Prior to the operation, an in depth disscusion with the patient, describing the anticipated degree of surgical difficult in completing a PN, the renal functional result, and a full description of potential complications including bleeding, infection, urinary fistula, the need for a prolonged perinephric drainage, and conversion to RN, if for technical reasons, a PN cannot be executed [58]. A preoperative nomogram is reassuring patients with small renal masses that a favorable long term prognosis is achievable with an effective resection [59]. A nephrometry scoring system (R.E.N.A.L) can categorize the degree of surgical difficulty for a planned PN [60]. The likelihood of a subsequent ipsi-lateral (<5%) or contra lateral (<5%) tumor recurrence in a patient's lifetime and the need for lifetime kidney imaging is discussed. For elderly patients with serious medical comorbidities and a small renal mass, active surveillance is the preferred alternative approach to PN since the vast majority of patients will not experience renal tumor progression in their remaining lifetimes and will die of competing causes [61,62].
The anticipated tempo of the hospital stay and postoperative recovery are described. The importance of walking on the first postoperative day (14 laps around the floor is a mile) to prevent deep venous thrombosis and deep breathing and incentive spirometry to prevent atelectasis and pneumonia is stressed. We rapidly progress patients to a regular diet with conversion to oral pain medication, and continued vigorous walking by the second day. The vast majority of patients are discharged on the second post op day. This regimen of vigorous walking continues at home with a switch to over the counter pain medications as soon as possible. We encourage 3 protein rich meals per day, continued walking at least 30 minutes twice per day with avoidance of heavy lifting for 3 months.
TECHNICAL FEATURES OF OPEN PARTIAL NEPHRECTOMY: SUPRA 11TH RIB MINI-FLANK SURGICAL INCISION
The traditional eleventh rib flank incision provided wide exposure but patients complained of significant postoperative pain, prolonged recovery, and for up to 50%, an uncomfortable and unsightly flank bulge usually caused by dennervated muscle [63] often with associated paresthesias and neuralgic pain. For the surgeon, rib resection and closure of this large incision also added significant operating time. These troublesome wound difficulties were major drivers for the development of laparoscopic RN for small renal tumors and a normal contra lateral kidney in the late 1990's.
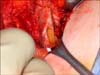
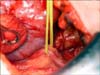
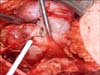
The "mini-flank" supra eleventh rib incision was developed as an effective alternative to this flank incision or laparoscopic RN [64]. With the patient in the standard flank position an 8-10 cm extraperitoneal incision is made between the bed of the 10th and 11th ribs (Fig. 1). Intercostal ligaments are sharply divided allowing for further separation of the ribs and rapid exposure to the retroperitoneum without rib resection (Fig. 2). The latissimus dorsi, external oblique and internal oblique muscles are transected and the transversus abdominus is divided in the direction of its fibers while preserving the intercostal neurovascular bundle. Using blunt dissection, the peritoneal cavity is mobilized medially, the perinpehric soft tissues laterally, and the diaphragmatic fibers and pleural superiorly. A small incision in the plane between the soft tissues overlying the psoas muscle and Gerota's fascia is then bluntly developed creating a flap of peritoneum that is retracted medially and exposes the kidney, ureter, and ipsilateral great vessel (vena cava on the right, aorta on the left). The Bookwalter retractor (Codman and Shurtleff Inc., Raynham, MA, USA) is placed using the bladder blade to medially retract the 10th rib and peritoneal flap superior medially which allows the kidney and perinephric soft tissues to move caudally into the wound. The short right-angle blade retracts the 11th rib laterally. Following blunt dissection of the intestinal contents, deeper malleable blades are placed to expose the great vessels. The ureter is isolated with a yellow vessel loop (Fig. 3) and division of lymphatic channels and soft tissues allow isolation of the renal artery and vein with red and blue vessel loops respectively (Fig. 4). We do not perform mass renal pedicle clamping with vascular clamps during cold ischemia. On the left side, the gonadal and adrenal veins may be ligated and divided to liberate the sometimes tethered renal vein to allow enhanced upward lifting of the kidney and improved access to the renal artery. The upward mobilization of the kidney decreases venous bleeding later during the tumor resection and facilitates identification and repair of rents in renal sinus veins. The upper pole of the kidney is separated from the adrenal using blunt dissection and perforating vessels are ligated and divided with the Ligasure (Covidien, Mansfield, MA. USA).
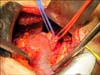
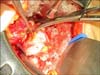
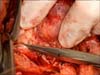
Palpation and visual and intraoperative US inspection of the renal surface is performed in order to confirm the location and depth of the tumor, identify any satellite lesions, and identify any branch renal vein or collecting system tumor invasion [22]. A polar or segmental artery may feed the exact tumor bearing area of the kidney and "regional ischemia" can be applied by placing a bulldog clamp on that artery allowing tumor resection while the rest of the kidney is normally perfused. For a purely exophytic tumor or a tumor in a patient with significant underlying CKD, resection of the tumor without renal artery occlusion is performed by gently applying a straight Satinsky clamp to the healthy kidney to allow for tumor resection and reconstruction in this alternative form of "regional ischemia" (Fig. 5). For other patients with large, endophytic, or perihilar tumors that require renal artery occlusion with a bulldog clamp, reno protective measures, including mannitol infusion (12.5 g/200 mL of normal saline) given 30 minutes prior to clamping and ice slush, are routinely used. It is unnecessary to place the kidney in a plastic bag prior to applying ice slush since the small surgical mini-flank incision does not cause significant hypothermia. We always apply ice slush hypothermia if renal artery occlusion is required and see no rationale in open PN for using warm ischemia alone. Following isolation of the tumor and its peritumoral fat, the renal cortex is scored with a 1-cm margin using the electrocautery and scissor dissection is utilized within the renal parenchymal plane (pink kidney tissue) (Fig. 6) with care not to get too close to the renal tumor's pseudo capsule (yellowish/golden tissue for conventional clear cell, brownish for oncocytoma, grayish for chromophobe tumors, yellowish/tan for papillary tumors). If dissection is too close to the tumor, readjustment to a deeper plane of dissection is made. We do not "enucleate" the tumor within its pseudocapsule. As the PN proceeds, 3-0 and 4-0 absorbable sutures are used to separately close any open small veins, arteries, and breaches in the collecting system that are encountered (Fig. 7). A search for venous bleeding can be accomplished by simply dropping the kidney into the wound (closer to the central venous pressure) and then raising it again. Deep suturing of the renal sinus is avoided because of concerns for iatrogenic a-v fistula or pseudo aneurysm formation and delayed bleeding events. The cut renal cortex is coagulated using the argon beam coagulator (ABC; Conmed Co., Utica, NY, USA) (Fig. 8). The surgical specimen is inspected to be certain there is a rim of surrounding renal and soft tissue and no fractures in the resection margin. The deep tumor surgical margin is marked with a silk suture to orient the specimen and then is delivered to the pathology department in sterile condition for a frozen section. Although the final pathology may take up to a week to complete, the frozen section can provide immediate reassurance to the surgeon that the resection was complete. Endophytic and renal sinus based tumors are especially challenging to locate and require correlation with preoperative CT and intraoperative US. Renal sinus tumors can emanate from the renal cortex facing the renal sinus and be attached by a small area of the cortex and otherwise float freely within the sinus without invasion of vascular or collecting system elements. The renal sinus can be accessed through a horizontal cortical incision in the avascular plane (Brodel's line). The tumor is identified on its lateral border and circumferentially dissected with a rim of surrounding cortex. Penfield or Cobb dissectors are used to gently lift the tumor out of the sinus with the final dissection of the actual renal cortical attachment performed sharply. The deep renal cortical attachment is marked with a silk suture as the deep margin and submitted to orient the specimen for pathologist. Surprisingly little collecting system reconstruction is required in many renal sinus tumor resections. During reconstruction within the sinus, care is taken to include renal papilla with a corresponding calyx and not occlude a calyx during suture repair that can lead to protracted leaks and or calyceal diverticuli.
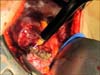
The resection cavity is filled with the hemostatic agents FloSeal (Baxter, Deerfield, IL, USA) and Surgicel (Johnson and Johnson, New Brunswick, NJ, USA) along with any available perinephric fat (Fig. 9). The renal capsule is then reapproximated using 0 chromic blunt tipped liver sutures pledgeted with Surgicel to prevent tearing of the renal cortical capsule and bleeding. The renal artery is unclamped and gentle pressure over resection bed is applied for 3-5 minutes. If no bleeding is observed and the collecting system was entered, a closed suction Jackson-Pratt drain (Allegiance Healthcare, McGaw Park, IL, USA) is placed through a separate stab wound in the retroperitoneal space posterior to the kidney. For exophytic tumors excised completely without entry into collecting system, the drain can be omitted. If venous oozing from the resection bed persists, another 5-minute period of gentle compression is applied. However, if brisk red arterial bleeding is observed careful inspection of the bed by cutting one or more liver sutures and ligation of any arterial bleeders is performed. Reclamping of the renal artery is avoided to prevent renal reperfusion injury. The surgical incision is closed in 2 layers using #1 polydioxanone and the skin reapproximated using 4-0 subcuticular absorbable sutures fashion (Fig. 10).
In the first report of 167 consecutive patients undergoing open mini-flank PN (n=133) or RN (n=34) from 2000-2003, excellent kidney exposure, decreased intraoperative estimated blood loss (EBL) (mean, 375 mL) and length of stay (mean, 4.5 days), and improved cosmetic results compared to traditional open techniques were obtained. After 18 months of follow-up, 3.6% of patients reported a bulge (no hernia but muscular atony) at the incision site, and 1 patient was diagnosed with an incisional hernia requiring surgical intervention [64]. In an update of 280 additional cases of open PNs, the median length of stay decreased further to 4 days, mean EBL was 300 mL, and a flank bulge was reported in only 1.8% of patients [65]. Muscle atony/bulge at the incision site without hernia is disconcerting for the patient and can be ameliorated or improved completely by exercises that twist the upper torso and core muscles (using an exercise bar, broom, or golf club). For the rare flank hernia, repair with synthetic mesh is more effective than primary closure which is likely to lead to recurrent hernia. With the advent of clinical post operative pathways emphasizing early ambulation, progression to regular diet and early switch to oral pain medications, length of stay for PN has been further reduced to 2.6 days [66].
COMPLEX APPLICATIONS OF PARTIAL NEPHRECTOMY
A challenging referral for PN, which in former years would have been automatic RN, are sporadic and hereditary bilateral and multifocal tumors, which have histological concordance in approximately 70% of cases and require careful surgical planning [67,68,69,70]. We approach the kidney with the largest tumor burden first since it carries the greater oncological threat [71]. The notion of performing the smaller PN first in order to "support" the larger contralateral tumor bearing kidney is no longer applicable since both can now usually be approached by PN. If the pathology of the larger is an indolent tumor with limited or no metastatic potential, such as renal oncocytosis or multifocal chromophobe renal cancer, a strong case for nonoperative management with careful observation alone for the contralateral renal tumor can be made. A careful balance between achieving local tumor control and maintaining renal function for as long as possible is paramount. In patients with diffuse multifocal tumors, such as renal oncocytosis [72] hereditary papillary renal cancer, or tuberous sclerosis, managed by long term surveillance of a tumor bearing kidney, eventual renal failure usually ensues, due to both macroscopic and microscopic disease progression and destruction of functioning glomeruli. Once the patient adjusts to dialysis, plans for completion RN of the nonfunctional kidney tumor bearing (or kidneys) can be made.
Multifocal tumors, many of which are minute in size, require careful inspection of the entire renal cortical surface along with intraoperative US to detect. As many of the minute tumors as possible are resected sharply either with fine scissors or a 15 scalpel blade with hemostasis obtained using the ABC. Larger multifocal tumors may require formal PN using cold ischemia [73]. During the resection of centrally located, hilar and perihilar tumors abutting proximal major renal arteries and veins, a complete resection, even if the surgical margin is close, should be attempted because a microscopically positive surgical margin does not translate into a local tumor recurrence or worse cancer specific survival [74]. For patients with a renal tumor in a solitary kidney, PN can achieve the dual goals of local tumor control and preservation of renal function. When prolonged renal artery ischemia and postoperative acute tubular necrosis is anticipated, a preoperative renal medicine consult and placement of a tunneled subclavian dialysis catheter is prudent [75,76,77] in case postoperative hemodialysis is required.
During PN an intrarenal muscular branched vein tumor thrombus may be encountered. This finding is associated with a worse prognosis than previously appreciated [78], yet PN can be done utilizing basic vascular surgical principles of proximal and distal vascular control and milking the thrombus back from the vein before dividing it. Similarly, with extension of a renal tumor into the collecting system [79], reconstructive urology principles, up to and including a pyeloplasty are utilized following the complete excision. Care must be taken during the reconstruction not to exclude a papilla from the renal calyceal system which can lead to a prolonged urinary fistula in the absence of retrograde pyelogram evidence of urinary extravasation. Most such leaks close spontaneously but may take weeks or months to do so. We generally do not place indwelling ureteral stents after collecting system repairs since postoperative urinary fistula can be accentuated by reflux of urine from the contralateral kidney. In the rare and difficult circumstance of resection of a tumor involving the majority of a low capacity intrarenal pelvis where pyeloplasty is no longer an option, closure of the proximal renal pelvis with ureteral calycostomy can achieve proper drainage from the kidney. In this case we place a double J ureteral stent and a closed suction drain.
For patients with recurrent sporadic or hereditary renal tumors following a prior PN, re-do PN can be difficult due to scar tissue involving the renal hilum and is associated with greater operative time, blood loss, and more complications. Meticulous dissection around the renal hilum can lead to a successful repeat PN in the vast majority of cases [80]. Treatment failures following either percutaneous or laparoscopic thermal ablation are now being referred for salvage PN. If the residual lesions are small and the patient is elderly, frail, or comorbidly ill, we recommend active surveillance which likely would have been our preference over thermal ablation initially. If the patient is young and otherwise well, PN is attempted. Patients are forewarned that PN may be unsuccessful due to extensive fibrosis and scarring with and conversion to RN a real possibility [81,82].
Medical oncologists may refer patients with metastatic renal cancer whose primary tumor is amenable to a cytoreductive PN. For patients with compromised renal function, PN makes sense, however, if the patient has sufficient renal functional reserve, a strong case can be made for a cytoreductive RN to avoid potential postoperative wound complications associated with the targeted agents that could delay the systemic chemotherapy. Long-term concerns for renal functional decline and potential late cardiovascular morbidity may not be as relevant in this patient population with a generally poor overall prognosis [83,84,85].
COMPLICATIONS OF OPEN PARTIAL NEPHRECTOMY
Complications related to PN generally fall in the three major categories of urinary fistula, bleeding, and infection. With more complex PN being performed, more complications will be encountered. If PN is performed without entry into the collecting system, urinary fistula is unlikely whereas for those tumors that are endophytic or within the renal sinus, postoperative urinary leakage is an expectation rather than complication. Variable definitions of urinary fistula in the literature (i.e., urinary leak for 2 days, 1 week, 2 weeks) can create confusion. When Memorial Sloan Kettering Cancer Center (MSKCC) investigators compared PN to RN and assessed complications with a graded scale, PN was not associated with more complications compared to RN but PN did have more procedure related complications (9% vs. 3%) due mainly to urinary fistula with reintervention rates of 2.5% for PN vs. 0.6% vs. RN. All but one reintervention involved endoscopic stents or placement of a percutaneous drain. Multivariate analysis indicated that operative time and solitary kidney were significantly associated with procedure related complications of PN [86]. Northwestern investigators analyzed 127 consecutive PN and reported urinary fistula rate of 13.3% with larger, endophytic tumors and those requiring collecting system repair more likely to have a prolonged leak [87]. MSKCC investigators analyzed 1,118 PN and defined persistent urinary leak as that lasting greater than 2 weeks or occurring in a patient that re-presents after drain removal with an urinoma requiring percutaneous drainage. Fifty-two patients developed a postoperative urinary fistula (4.4%) with persistent leak accounting for 4% and delayed fistula presentation accounting for 0.4% of cases. Factors associated with urine leak were larger tumors (3.5 cm vs. 2.6 cm), more blood loss (400 mL vs. 300 mL), and longer ischemia time (50 minutes vs. 39 minutes). Overall, 36 patients (69%) had resolution of the fistula without intervention while 16 patients (31%) underwent a stent (n=8) and another drain (n=2). No patient required a nephrectomy to manage the fistula [88]. Although most urologists use closed sunction drains when necessary, there is no real data that they are any more effective than Penrose drains [89]. In the unfortunate circumstance of persistent leaks over 6-8 weeks despite good nutritional support, a cystoscopy and retrograde pyelogram should be done to exclude a distal ureteral obstruction (stenosis or ureteral kink) or injury. A disconcerting finding on such a study is a normal pyelocalyceal system yet persistent urinary drainage which indicates an excluded renal papilla without a corresponding calyx. The leak may take several months to resolve until that papilla is no longer functional. If a PN is performed and no elements of the collecting system are entered, it is perfectly safe to complete the operation without the placement of a drain which will alleviate drain related patient discomfort and decrease the likelihood of a drain related nosocomial infection [90].
Pseudo aneurysm or iatrogenic arterial venous fistula forms when surgical repair of vascular rents fuses arteries and veins with direct communication developing between the two. On rare occasions, a palpable thrill can be noticed over the resection site at the end of a PN. In this case, immediate re-exploration of the surgical bed with ligation of the communicating vessels or completion RN is performed. These can present with delayed postoperative bleeding, perinephric hematoma with pain, ureteral colic, gross hematuria, hypotension, flank mass, or flank discoloration from dissecting blood. Following appropriate resuscitation, CT renal protocol is performed which will reveal arterial contrast pooling with or without perinephric hematoma, a finding which should initiate an urgent request for selective renal artery angiogram and coil embolization. The interventional radiologist should make every effort to occlude tertiary and quaternary branches of the renal artery as close to pseudo aneurysmal pocket as possible in order to limit collateral damage to viable, healthy renal tissue. MSKCC investigators reported 1,461 PN performed from 2003-2010. There were 15 pseudo aneurysms (1%) including 7/1,160 (0.6%) in open PN and 8/301 (2.6%) minimally invasive PN. Fourteen of fifteen patients were successfully treated with embolization but one patient with a coagulapathy required a completion RN [91].
FOLLOW-UP AFTER PARTIAL NEPHRECTOMY FOR CLINICALLY LOCALIZED RENAL NEOPLASMS
In an attempt to standardize the follow-up after nephrectomy, the AUA recently published guidelines based on tumor stage. All patients should undergo interval history and physical examination and laboratory testing including blood urea nitrogen/creatinine, urinalysis and eGFR. Low risk patients (pT1, N0, Nx) should undergo a baseline abdominal scan (CT or MRI) within 3-12 months following surgery. If the initial scan is negative, abdominal imaging (US, CT, or MRI) may be performed yearly for 3 years, based on the individual risk for recurrence. In addition, low-risk patients are recommended to undergo yearly chest x-ray to asses for pulmonary metastases for 3 years, and if clinically indicated beyond that period. Moderate to high risk patients (pT2-4N0 or any N+ stage) should undergo baseline chest and abdominal scans (CT or MRI) within 3-6 months following surgery, with continued imaging every 6 months for 3 years at least, and annually thereafter to year 5. Imaging beyond 5 years may be performed at the discretion of the clinician [92]. At MSKCC, after two years without evidence of disease recurrence, we refer patients to our "survivorship" clinic where long term renal cancer follow-up, general medical care, and oncological screening is provided. As much as possible, renal US and chest x-ray are utilized in follow-up unless specific patient signs or symptoms need to be directly addressed.
CONCLUSIONS
PN is an essential operation for the management of small renal masses increasingly detected by cross sectional imaging usually obtained for the evaluation of nonspecific abdominal or musculoskeletal complaints. With the median size of <4 cm, PN provides equivalent oncological control for the T1 tumor as does RN while at the same time preventing or delaying CKD and its potential for late cardiovascular morbidity and mortality. This kidney functional preserving effect is amplified by the fact that approximately 45% of patients will have a benign or indolent tumor resected with little or no metastatic potential. Open PN using a mini-flank supra 11th rib approach is a highly effective operation that should be part of every renal surgeon's arsenal. This approach provides rapid exposure to the kidney is useful for both straightforward and complex operations, is cost effective, and now associated with a surgical length of stay 2.6 days. Potential complications of postoperative bleeding, infection, and urinary fistula can occur but most are managed with conservative measures that ultimately preserve the kidney. The mini-flank approach can readily be taught to residents and fellows and can potentially increase the pool of patients worldwide that can undergo a kidney sparing operation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download