1. Buonsenso D, Cataldi L. Urinary tract infections in children: a review. Minerva Pediatr. 2012; 64:145–157.
2. Montini G, Tullus K, Hewitt I. Febrile urinary tract infections in children. N Engl J Med. 2011; 365:239–250.
3. Elder JS. Urinary tract infection. In : Kliegman RM, Stanton BF, St. Geme JW, Schor NF, Behrman RE, editors. Nelson textbook of pediatrics. 19th ed. Philadelphia: Saunders;2011. p. 1829–1838.
4. Salo J, Ikaheimo R, Tapiainen T, Uhari M. Childhood urinary tract infections as a cause of chronic kidney disease. Pediatrics. 2011; 128:840–847.
5. Bhat RG, Katy TA, Place FC. Pediatric urinary tract infections. Emerg Med Clin North Am. 2011; 29:637–653.
6. Ayazi P, Mahyar A, Hashemi HJ, Daneshi MM, Karimzadeh T, Salimi F. Comparison of procalcitonin and C-reactive protein tests in children with urinary infection. Iran J Pediatr. 2009; 19:381–386.
7. Mattoo TK. Vesicoureteral reflux and reflux nephropathy. Adv Chronic Kidney Dis. 2011; 18:348–354.
8. Ayazi P, Moshiri SA, Mahyar A, Moradi M. The effect of vitamin A on renal damage following acute pyelonephritis in children. Eur J Pediatr. 2011; 170:347–350.
9. Leonardo CR, Filgueiras MF, Vasconcelos MM, Vasconcelos R, Marino VP, Pires C, et al. Risk factors for renal scarring in children and adolescents with lower urinary tract dysfunction. Pediatr Nephrol. 2007; 22:1891–1896.
10. Bensman A, Dunand O, Ulinski T. Urinary tract infection. In : Avner ED, Harman WE, Niaudet P, Yoshikawa N, editors. Pediatric nephrology. 6th ed. Berlin: Springer;2009. p. 1007–1025.
11. Cleper R, Krause I, Eisenstein B, Davidovits M. Prevalence of vesicoureteral reflux in neonatal urinary tract infection. Clin Pediatr (Phila). 2004; 43:619–625.
12. Greenbaum LA, Mesrobian HG. Vesicoureteral reflux. Pediatr Clin North Am. 2006; 53:413–427.
13. Williams G, Fletcher JT, Alexander SI, Craig JC. Vesicoureteral reflux. J Am Soc Nephrol. 2008; 19:847–862.
14. Goonasekera CD, Abeysekera CK. Vesicoureteric reflux and reflux nephropathy. Indian J Pediatr. 2003; 70:241–249.
15. Sjöström S, Sillen U, Jodal U, Sameby L, Sixt R, Stokland E. Predictive factors for resolution of congenital high grade vesicoureteral reflux in infants: results of univariate and multivariate analyses. J Urol. 2010; 183:1177–1184.
16. Soylu A, Kasap B, Demir K, Turkmen M, Kavukcu S. Predictive value of clinical and laboratory variables for vesicoureteral reflux in children. Pediatr Nephrol. 2007; 22:844–848.
17. Lebowitz RL, Olbing H, Parkkulainen KV, Smellie JM, Tamminen-Mobius TE. International system of radiographic grading of vesicoureteric reflux: International Reflux Study in children. Pediatr Radiol. 1985; 15:105–109.
18. Montini G, Zucchetta P, Tomasi L, Talenti E, Rigamonti W, Picco G, et al. Value of imaging studies after a first febrile urinary tract infection in young children: data from Italian renal infection study 1. Pediatrics. 2009; 123:e239–e246.
19. American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on Urinary Tract Infection. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics. 1999; 103:843–852.
20. Kass EJ, Kernen KM, Carey JM. Paediatric urinary tract infection and the necessity of complete urological imaging. BJU Int. 2000; 86:94–96.
21. Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003; 348:195–202.
22. Oostenbrink R, van der Heijden AJ, Moons KG, Moll HA. Prediction of vesico-ureteric reflux in childhood urinary tract infection: a multivariate approach. Acta Paediatr. 2000; 89:806–810.
23. Leroy S, Marc E, Adamsbaum C, Gendrel D, Breart G, Chalumeau M. Prediction of vesicoureteral reflux after a first febrile urinary tract infection in children: validation of a clinical decision rule. Arch Dis Child. 2006; 91:241–244.
24. Tseng MH, Lin WJ, Lo WT, Wang SR, Chu ML, Wang CC. Does a normal DMSA obviate the performance of voiding cystourethrography in evaluation of young children after their first urinary tract infection? J Pediatr. 2007; 150:96–99.
25. Camacho V, Estorch M, Fraga G, Mena E, Fuertes J, Hernandez MA, et al. DMSA study performed during febrile urinary tract infection: a predictor of patient outcome? Eur J Nucl Med Mol Imaging. 2004; 31:862–866.
26. Lee HY, Soh BH, Hong CH, Kim MJ, Han SW. The efficacy of ultrasound and dimercaptosuccinic acid scan in predicting vesicoureteral reflux in children below the age of 2 years with their first febrile urinary tract infection. Pediatr Nephrol. 2009; 24:2009–2013.
27. Sorkhi H, Nooreddini HG, Amiri M, Osia S, Farhadi-Niakee S. Prediction of vesicoureteral reflux in children with first urinary tract infection by dimercaptosuccinic acid and ultrasonography. Iran J Pediatr. 2012; 22:57–62.
28. Leroy S, Adamsbaum C, Marc E, Moulin F, Raymond J, Gendrel D, et al. Procalcitonin as a predictor of vesicoureteral reflux in children with a first febrile urinary tract infection. Pediatrics. 2005; 115:e706–e709.
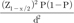 Consecutive sampling continued until the desired sample size was reached.
Consecutive sampling continued until the desired sample size was reached.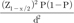 Consecutive sampling continued until the desired sample size was reached.
Consecutive sampling continued until the desired sample size was reached.


