Abstract
Purpose
To assess changes in lower urinary tract symptoms (LUTS), prostate volume, and serum prostate-specific antigen (PSA) after discontinuation of 5-alpha reductase inhibitor (5ARI) combination therapy in patients with benign prostatic hyperplasia (BPH).
Materials and Methods
From December 2003 to December 2012, data were collected retrospectively from 81 men more than 40 years of age with moderate to severe BPH symptoms (International Prostate Symptom Score [IPSS]≥8). The men were classified into group 1 (n=42) and group 2 (n=39) according to the use of 5ARI therapy. A combination of dutasteride 0.5 mg with tamsulosin 0.2 mg was given daily to all patients for 1 year. For the next 1 year, group 1 (n=42) received the combination therapy and group 2 (n=39) received tamsulosin 0.2 mg monotherapy only. The IPSS, prostate volume, and PSA level were measured at baseline and at 12 and 24 months according to the use of dutasteride.
Results
Discontinuation of dutasteride led to significant deterioration of LUTS, increased prostate volume, and increased PSA level. The repeated-measures analysis of variance showed that the changes in IPSS, prostate volume, and PSA level over time also differed significantly between groups 1 and 2 (p<0.001).
Conclusions
Withdrawal of 5ARI during combination therapy resulted in prostate regrowth and deterioration of LUTS. The PSA level is also affected by the use of 5ARI. Therefore, regular check-up of the IPSS and PSA level may be helpful for all patients who either continue or discontinue the use of 5ARI.
Benign prostatic hyperplasia (BPH) is a progressive and androgen-dependent disease that causes lower urinary tract symptoms (LUTS) [1]. Although BPH is not a life-threatening disease, it can have a negative effect on the quality of life [2].
Alpha-adrenergic receptor antagonists (α-blockers) and 5-alpha reductase inhibitor (5ARI) are the first-line treatment option in patients with BPH [3]. 5ARI reduces prostate volume and is approved for the treatment of clinical BPH [4]. 5ARI blocks the conversion of the sex steroid hormone testosterone to dihydrotestosterone (DHT), which is an essential component of BPH pathogenesis [5]. Previous studies reported that the serum prostate-specific antigen (PSA) level and prostate volume were decreased by approximately 50% and 20%, respectively, after treatment with 5ARI [6]. However, some clinical questions remain as to how to interpret the PSA level in men treated with 5ARI [5]. Furthermore, the effects on LUTS and prostate volume after discontinuation of 5ARI are not well understood. Therefore, in the present study, we analyzed the changes in LUTS, prostate volume, and PSA level after discontinuation of 5ARI.
From December 2003 to December 2012, 81 men more than 40 years of age with moderate to severe BPH symptoms as determined by the International Prostate Symptom Score (IPSS≥8) and a prostate volume of greater than 25 cm3 measured by transrectal ultrasonography (TRUS) were collected retrospectively for the study. Two patients (case 1, 4.91 ng/mL; case 2, 5.43 ng/mL) with a PSA level of 4 or more and with a negative pathologic result on the initial prostate biopsy were included in the study. Patients were excluded from the study if they had chronic urinary tract infection; a history of prostate or testicular surgery, prostate cancer, acute urinary retention, or chronically large postvoid residual urine volume; or 5ARI treatment history.
The patients were classified into group 1 (n=42) and group 2 (n=39) according to 5ARI use. A combination of dutasteride 0.5 mg with tamsulosin 0.2 mg was given daily to all patients for 1 year. Then, these patients were divided into 2 groups. For the next 1 year, group 1 (n=42) received a combination of dutasteride 0.5 mg with tamsulosin 0.2 mg daily, and group 2 (n=39) received α-blocker monotherapy only. The IPSS, prostate volume, and PSA level were measured at baseline and at 12 and 24 months. With TRUS (Aloka, Tokyo, Japan), prostate volume was measured by use of the prostate ellipsoid formula and was calculated by multiplying the longest anteroposterior (height, H), transverse (width, W), and cephalocaudal (length, L) diameters and 0.524 (H×W×L×π/6).
PASW ver. 18.0 (IBM Co., Armonk, NY, USA) was used for all statistical analyses. Student t-test was used to compare the baseline values of IPSS, prostate volume, and serum PSA level in each group. The repeated-measures analysis of variance was performed to compare the change in IPSS, prostate volume, and PSA level from 0 to 12 months and also from 12 months to 24 months. A p-value of less than 0.05 was considered statistically significant.
The mean age of the patients (n=81) was 71.70 years (standard deviation [SD], 7.80). The total IPSS was 16.53 (SD, 7.70). The mean prostate volume was 43.96 cm3 (SD, 17.38) and the mean baseline serum PSA level was 3.00 ng/mL (SD, 1.98). The patients were classified into group 1 (n=42) and group 2 (n=39). There were no statistically significant differences between the two groups in mean age, total IPSS, baseline prostate volume, or PSA level (Table 1).
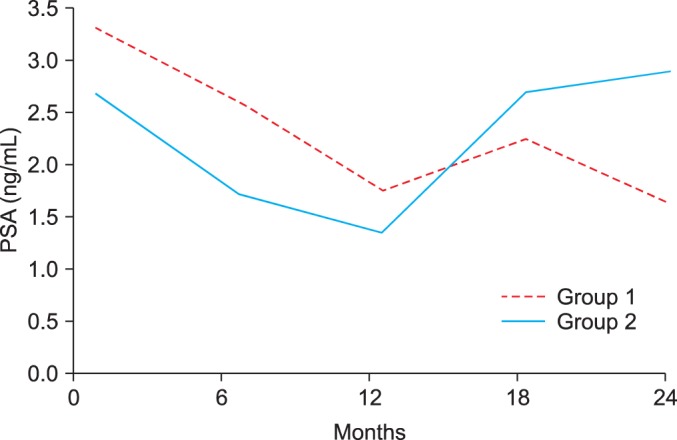
The differences in total IPSS, prostate volume, and serum PSA level according to the absence of dutasteride are shown in Table 2. At 1 year after combination therapy, total IPSS had decreased by 17.6% (from 17.88 [SD, 7.77] to 14.95 [SD, 7.27]; p<0.001) in group 1 and by 13.3% (from 15.50 [SD, 7.60] to 13.45 [SD, 2.50]; p<0.001) in group 2. However, at 1 year after dutasteride had been withdrawn, total IPSS had increased by up to 7.6% (from 13.45 [SD, 2.50] to 14.93 [SD, 5.48]; p<0.001) in group 2 (Table 2).
At 1 year after combination therapy, the prostate volume was reduced by 21.9% (from 41.59 cm3 [SD, 13.97] to 32.95 cm3 [SD, 10.10]; p<0.001) in group 1 and by 15.5% (from 45.96 cm3 [SD, 23.02] to 38.96 cm3 [SD, 23.91]; p<0.001] in group 2. At 1 year after dutasteride had been withdrawn, the prostate volume had increased by up to 13.1% [from 38.96 (23.91) cm3 to 43.82 (23.45) cm3; p<0.001]. At 1 year after combination therapy, the transition zone volume had been reduced by 20.0% (from 15.62 cm3 [SD, 5.76] to 12.23 cm3 [SD, 4.50]; p<0.001] in group 1 and by 17.6% (from 17.84 cm3 [SD, 10.35] to 14.61 cm3 [SD, 9.81]; p<0.001] in group 2. At 1 year after dutasteride had been withdrawn, the prostate volume had increased by up to 14.2% (from 14.61 cm3 [SD, 9.81] to 16.46 cm3 [SD, 10.06]; p<0.001] in group 2.
The PSA level had decreased by 48.4% (from 3.30 ng/mL [SD, 2.09] to 1.75 ng/mL [SD, 1.33]; p<0.001) in group 1 and by 49.4% (from 2.67 ng/mL [SD, 1.82] to 1.35 ng/mL [SD, 0.92]; p<0.001) in group 2. However, 1 year after discontinuation of dutasteride, the PSA level had increased up to 108.2% of the baseline level (from 1.35 ng/mL [SD, 0.92] to 2.89 ng/mL [SD, 2.10]; p<0.001) in group 2 (Fig. 1). The changes in IPSS, prostate volume, and PSA over time also did not differ significantly between groups 1 and 2 (Table 2).
Patients discontinued dutasteride in group 2 owing to adverse events, including loss of libido (n=9) or erectile dysfunction (n=17), lack of therapy efficacy (n=7), abnormal ejaculation (n=5), and gynecomastia (n=1). No patient developed acute urinary retention or required disease-related surgery.
Many senile men develop obstructive and irritative LUTS. These symptoms may be associated with BPH, which causes extrinsic compression of the prostatic urethra leading to impaired voiding [7,8]. BPH is a common disease in aging men that leads to deterioration in symptoms, acute urinary retention, BPH-related prostatic surgery, gross hematuria, urinary tract infection, bladder stones, and renal insufficiency [9]. Men who have a prostate volume of more than 30 mL are more likely to have moderate-to-severe symptoms (3.5 times), decreased flow rates (2.5 times), and acute urinary retention (3-4 times) than are men with a prostate volume of less than 30 mL [10]. BPH can significantly affect quality of life in a negative direction, thus requiring treatment.
BPH can be managed medically with alpha-blockers, 5-ARI, or a combination of both [11,12]. Alpha-blockers relax the smooth-muscle tone in the prostate and bladder neck [13]. Alpha-blockers have a rapid onset of relief of urinary symptoms. However, alpha-blockers do not provide long-term reduction in the risk of acute urinary retention or BPH-related surgery [8]. The main role of 5ARI is to induce glandular epithelial atrophy, which causes volume reduction in the prostate [14]. 5ARI is responsible for impeding the conversion of testosterone to DHT by inhibiting the nuclear-bound steroid 5α-reductase (5AR) isoenzymes [15,16]. Both normal prostate development and hyperplasia of the prostatic transitional zone are regulated by DHT [16]. 5ARI can improve symptoms and can have a preventive effect on BPH progression. Men taking 5ARI have a 57% reduction in the risk of acute urinary retention compared with those taking placebo and a 34% reduction in the risk of surgery [17]. Dutasteride is a potent inhibitor of both types 1 and 2 5ARs. Dutasteride results in a reduction of 20% or more in the size of the prostate and a reduction of 48% in the risk of BPH-related surgery [5,10]. In our study, total IPSS improvement and prostate volume reduction were evident in all men after treatment with dutasteride 0.5 mg [18].
The optimal duration of 5ARI treatment has not been clearly defined. Few reports have discussed the effect of discontinuation of 5ARI on LUTS, prostate volume, and PSA level. Stoner [19] demonstrated that the prostate volume returned to near the baseline values at 12 weeks after discontinuation of 5ARI. In our study, the mean percentage of prostate regrowth had reached 95.5% of the baseline prostate volume 1 year after discontinuation of dutasteride. Owing to the complex etiologies of prostate enlargement, the mechanism of prostate regrowth after 5ARI withdrawal is not fully understood. The hyperplastic changes in BPH are an androgen-dependent process, and it is possible that the sensitivity to DHT is increased or overly activated as a result of the prolonged pharmacologic inhibition of 5α-reductase by the 5ARI [20]. Androgen receptors, which become more sensitive or up-regulated during treatment with 5ARI, may be considered as an alternative mechanism [20]. They can affect regrowth even after discontinuation of the inhibition of the 5α-reductase. Moreover, prostate regrowth is associated with deterioration of urinary symptoms. Our study showed that the total IPSS had increased 1 year after cessation of dutasteride. Therefore, we propose that reuse of 5ARI is helpful in cases that have aggravated BPH symptoms or complications of BPH.
The PSA level is also commonly used as a screening tool for prostate cancer, and the level of 4.0 ng/mL or greater, or more recently, greater than 2.5 ng/mL, has predictive value for prostate cancer detection [21]. PSA velocity also has some predictive potency for detection of the cancer, especially in men with a PSA level of 4.0 ng/mL or less. The epithelial cells of the prostate secrete PSA, and it is synthesized by androgen regulation [22]. At 1 year of 5ARI treatment, the treatment results in an approximate 50% decrease in the PSA level compared with the baseline serum level [23]. The use of 5ARI changes the PSA level, and this may distract the clinician's attention. The PSA level must be multiplied by 2 to maintain its usefulness as a marker [18,24]. The PSA level increases after discontinuation of 5ARI because PSA is secreted by the epithelial cells of the prostate. In our study, the PSA level had decreased 1 year after combination therapy and had increased 1 year after discontinuation of 5ARI. In group 1, two patients had continued PSA level elevation, and they were finally diagnosed as having prostate cancer. Thus, regular monitoring of the PSA level may be helpful for prostate cancer detection. Although it is not mandatory, we suggest measuring prostate volume by using TRUS in selected patients with aggravated BPH symptoms or a high PSA level. TRUS can provide PSA density, which helps clinicians make decisions about further evaluations such as prostate biopsy.
The limitations of this study include it being a retrospective review and having a small number of cases. Prospective randomized clinical trials with a larger number of cases are needed to fully determine the effect of discontinuing 5ARI. Maximal urinary flow rate is needed to evaluate the changes in urinary symptoms with the use of 5ARI. Last, our study investigated changes 1 year after the discontinuation of dutasteride, but longer follow-up is necessary to make therapeutic decisions about the effect of 5ARI.
Our study showed that withdrawal of 5ARI during combination therapy resulted in prostate regrowth and deterioration of LUTS. The PSA level was reduced by the use of 5ARI, but discontinuation of the drug resulted in an increase in the PSA level. As a result, regular checkup of the IPSS and PSA level may be helpful for all patients who continue or discontinue use of 5ARI. We recommend measuring prostate volume by using TRUS in selected patients with aggravated BPH symptoms or a high PSA level.
References
1. Emberton M, Andriole GL, de la Rosette J, Djavan B, Hoefner K, Vela Navarrete R, et al. Benign prostatic hyperplasia: a progressive disease of aging men. Urology. 2003; 61:267–273. PMID: 12597928.

2. Jacobsen SJ, Jacobson DJ, Girman CJ, Roberts RO, Rhodes T, Guess HA, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997; 158:481–487. PMID: 9224329.

3. Boyle P, Roehrborn C, Harkaway R, Logie J, de la Rosette J, Emberton M. 5-Alpha reductase inhibition provides superior benefits to alpha blockade by preventing AUR and BPH-related surgery. Eur Urol. 2004; 45:620–626. PMID: 15082205.

4. Debruyne F, Barkin J, van Erps P, Reis M, Tammela TL, Roehrborn C, et al. Efficacy and safety of long-term treatment with the dual 5 alpha-reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. Eur Urol. 2004; 46:488–494. PMID: 15363566.
5. Marks LS, Andriole GL, Fitzpatrick JM, Schulman CC, Roehrborn CG. The interpretation of serum prostate specific antigen in men receiving 5alpha-reductase inhibitors: a review and clinical recommendations. J Urol. 2006; 176:868–874. PMID: 16890642.
6. Madersbacher S, Marszalek M, Lackner J, Berger P, Schatzl G. The long-term outcome of medical therapy for BPH. Eur Urol. 2007; 51:1522–1533. PMID: 17416456.

7. Roehrborn CG. 5-Alpha-reductase inhibitors prevent the progression of benign prostatic hyperplasia. Rev Urol. 2003; 5(Suppl 4):S18–S27. PMID: 16985959.
8. Tanguay S, Awde M, Brock G, Casey R, Kozak J, Lee J, et al. Diagnosis and management of benign prostatic hyperplasia in primary care. Can Urol Assoc J. 2009; 3(3 Suppl 2):S92–S100. PMID: 19543429.

9. Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G. ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002; 60:434–441. PMID: 12350480.

10. Marberger M, Harkaway R, de la Rosette J. Optimising the medical management of benign prostatic hyperplasia. Eur Urol. 2004; 45:411–419. PMID: 15041103.

11. Nickel JC, Barkin J, Koch C, Dupont C, Elhilali M. Finasteride monotherapy maintains stable lower urinary tract symptoms in men with benign prostatic hyperplasia following cessation of alpha blockers. Can Urol Assoc J. 2008; 2:16–21. PMID: 18542722.

12. Roehrborn CG, Lukkarinen O, Mark S, Siami P, Ramsdell J, Zinner N. Long-term sustained improvement in symptoms of benign prostatic hyperplasia with the dual 5alpha-reductase inhibitor dutasteride: results of 4-year studies. BJU Int. 2005; 96:572–577. PMID: 16104912.

13. Kirby RS, Roehrborn C, Boyle P, Bartsch G, Jardin A, Cary MM, et al. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology. 2003; 61:119–126. PMID: 12559281.

14. McConnell JD, Roehrborn CG, Bautista OM, Andriole GL Jr, Dixon CM, Kusek JW, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003; 349:2387–2398. PMID: 14681504.

15. Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab. 2004; 89:2179–2184. PMID: 15126539.
16. Barkin J, Guimaraes M, Jacobi G, Pushkar D, Taylor S, van Vierssen Trip OB. Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5alpha-reductase inhibitor dutasteride. Eur Urol. 2003; 44:461–466. PMID: 14499682.
17. Andersen JT, Nickel JC, Marshall VR, Schulman CC, Boyle P. Finasteride significantly reduces acute urinary retention and need for surgery in patients with symptomatic benign prostatic hyperplasia. Urology. 1997; 49:839–845. PMID: 9187688.

18. Kaplan SA, Ghafar MA, Volpe MA, Lam JS, Fromer D, Te AE. PSA response to finasteride challenge in men with a serum PSA greater than 4 ng/ml and previous negative prostate biopsy: preliminary study. Urology. 2002; 60:464–468. PMID: 12350485.

19. Stoner E. The clinical effects of a 5 alpha-reductase inhibitor, finasteride, on benign prostatic hyperplasia. The Finasteride Study Group. J Urol. 1992; 147:1298–1302. PMID: 1373779.
20. Jeong YB, Kwon KS, Kim SD, Kim HJ. Effect of discontinuation of 5alpha-reductase inhibitors on prostate volume and symptoms in men with BPH: a prospective study. Urology. 2009; 73:802–806. PMID: 19193422.
21. Andriole GL, Marberger M, Roehrborn CG. Clinical usefulness of serum prostate specific antigen for the detection of prostate cancer is preserved in men receiving the dual 5alpha-reductase inhibitor dutasteride. J Urol. 2006; 175:1657–1662. PMID: 16600723.
22. Bartsch G, Fitzpatrick JM, Schalken JA, Isaacs J, Nordling J, Roehrborn CG. Consensus statement: the role of prostate-specific antigen in managing the patient with benign prostatic hyperplasia. BJU Int. 2004; 93(Suppl 1):27–29. PMID: 15009083.

23. Guess HA, Gormley GJ, Stoner E, Oesterling JE. The effect of finasteride on prostate specific antigen: review of available data. J Urol. 1996; 155:3–9. PMID: 7490873.

24. Andriole GL, Bostwick D, Brawley OW, Gomella L, Marberger M, Montorsi F, et al. The effect of dutasteride on the usefulness of prostate specific antigen for the diagnosis of high grade and clinically relevant prostate cancer in men with a previous negative biopsy: results from the REDUCE study. J Urol. 2011; 185:126–131. PMID: 21074214.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download