Abstract
Purpose
It is debated whether treatment delay worsens oncologic results in localized prostate cancer (PCa). Few studies have focused on the role of a delay between the time of biopsy and the time of surgery. Thus, we aimed to investigate the effect of the time period between biopsy and surgery on Gleason score upgrading (GSU).
Materials and Methods
A total of 290 patients who underwent radical retropubic prostatectomy in Ankara Training and Research Hospital were included in the study. The biopsy Gleason score, age, total prostate-specific antigen (PSA) value, prostate volumes, and PSA density (PSAD) were analyzed in all patients. The patients were divided into two groups: patients with GSU (group 1) and patients without GSU (group 2). Variables having a p-value of ≤0.05 in the univariate analysis were selected and then evaluated by use of multivariate logistic regression models. Results were considered significant at p<0.05.
Results
GSU occurred in 121 of 290 patients (41.7%). The mean age of the patients was 66.0±7.2 years in group 1 and 65.05±5.60 years in group 2 (p=0.18). The mean PSA values of groups 1 and 2 were 8.6±4.1 and 8.8±4.3 ng/dL, respectively. The mean prostate volumes of groups 1 and 2 were 43.8±14.1 and 59.5±29.8 mL, respectively. The PSAD of group 1 was significantly higher than that of group 2 (0.20 vs. 0.17, p=0.003). The mean time to surgery was shorter in group 2 (group 1, 52.2±22.6 days; group 2, 45.3±15.5 days; p=0.004). According to the logistic regression, time from biopsy to surgery is important in the prediction of GSU.
At present, the diagnosis rate of early-stage prostate cancer (PCa) has increased owing to widely used prostate-specific antigen (PSA) measurements [1]. The treatment options of newly diagnosed, low-risk localized PCa include active follow-up, radical prostatectomy (RP), and radiotherapy [2,3]. Gleason score (GS) at biopsy is one of the most important factors for deciding on a treatment. Unfortunately, a clear discordance has been shown between biopsy and pathologic GS. Several studies have reported upgrading from GS6 at the time of biopsy to GS7 after RP in up to 30% to 60% of cases [4,5].
Several factors affect the duration between biopsy and surgery in patients who are planning to undergo RP. Among these are increased use of active surveillance, the time taken to inform patients about the therapeutic options, and scheduling issues. It is debated whether treatment delay deteriorates oncologic results in localized PCa [6,7,8]. On the other hand, Gleason score upgrading (GSU) at prostatectomy has been associated with poorer outcomes [9].
After Institutional Review Board approval was obtained for the study, 422 patients who underwent radical retropubic prostatectomy in Ankara Training and Research Hospital between August 2005 and May 2012 were retrospectively reviewed. A total of 132 patients with PSA>20 ng/dL, a clinical stage greater than T1c, biopsy GS>6, missing data, and a history of previous biopsy were excluded. The remaining 290 patients were included in the study. GSU was regarded as a GS equal to or greater than 7 after a biopsy GS of 5 or 6. The pathology specimens were evaluated by a single experienced genitourinary pathologist of the hospital mentioned above. The patients were divided into two groups as those with GSU (group 1) and those without GSU (group 2).
All patients included in the study had normal results on a digital rectal examination. All patients were diagnosed with PCa after 12-core biopsies obtained with transrectal ultrasonography (TRUS), which was performed because of a high PSA level (>2.5 ng/dL).
The biopsy GS, age, total PSA value, prostate volumes measured with TRUS, PSA density (PSAD), the number of positive cores, the mean tumor percentage in the cores, mean biopsy core length, pathologic GS, postoperative tumor volume, presence of capsule invasion, presence of positive surgical margins, seminal vesicle invasion, positive lymph nodes, and the duration between diagnosis of PCa with biopsy and the time of RP were analyzed in all patients.
TRUS was performed by using LOG-13 41123WS1 (General Electric, New York, NY, USA) ultrasonography equipment with a 6.5-MHz biplane transrectal probe. Transrectal ultrasound prostate volume was calculated by using a computer-generated elliptical estimation of 0.52×length×width×height.
The data analysis was performed by using SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA). Descriptive statistics for variables with a normal distribution were shown as means±standard deviations. Statistical analysis of continuous data between two groups consisted of Student t-test. Categorical variables between the two groups were tested with the chi-square and Fisher exact test.
Variables having a p-value of ≤0.05 in the univariate analysis (prostate volume, PSAD, pathological tumor volume, time from biopsy to surgery, and mean percentage of tumor in cores) were selected and then evaluated by multivariate logistic regression models. The significant factors were listed with corresponding odds ratios. All results were considered significant at p<0.05.
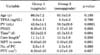
GSU occurred in 121 of 290 patients (41.7%). The mean age of the patients was 66.0±7.2 years in group 1 and 65.05± 5.60 years in group 2 (p=0.18). The mean prostate volumes of groups 1 and 2 were 43.8±14.1 mL and 59.5±29.8 mL, respectively. The PSAD of group 1 was significantly higher than that of group 2 (0.20 vs. 0.17, respectively; p=0.003) (Table 1). The average percentages of tumor in cores were 0.40%±0.29% and 0.30%±0.22% in groups 1 and 2, respectively (p=0.001) (Table 1).
There was capsule invasion in 74 of 121 patients in group 1 (61.1%), whereas capsule invasion was evident in 34 of 169 patients in group 2 (20.1%, p=0.0001). Similarly, extraprostatic extension was seen in 24 of 121 patients in group 1 (19.8%) and 11 of 169 patients (6.5%) in group 2 (p=0.001). The rates of seminal vesicle invasion in groups 1 and 2 were 4.1% and 1.7%, respectively (p=0.22). The categorical comparative data of groups 1 and 2 are presented in Table 2. The mean time to surgery was shorter in group 2 (group 1, 52.2±22.6 days; group 2, 45.3±15.5 days; p=0.004).
According to the logistic regression, the time from biopsy to surgery, prostate volume, and percentage of tumor in cores were important in the prediction of GSU with a receiver operating characteristic area under the curve of 0.737 (95% confidence interval, 0.680-0.793; p=0.0001). The significant factors are listed with corresponding odds ratios (Exp(B)) in Table 3.
In today's PSA era, most PCa cases are diagnosed when they have a low risk and are at a low stage. In case of newly diagnosed, clinically localized PCa, informing patients about their therapeutic options takes some time. The time elapsed for this information differs for every patient, and informing the patient can take a long time.
It is debated whether delay in treatment has a negative effect on oncological results [6,7,8]. Studies that have analyzed the effect of the period between the biopsy and the surgery usually take biochemical failure (BF) into account, because it is the earliest marker. The results of these studies are conflicting, however [7,8].
Nam et al. [7] reported that the 10-year recurrence-free survival rate was 74.6% when the period between diagnosis and RP was less than 3 months, whereas it was 61.3% when this period was more than 3 months. O'Brien et al. [13] performed a study on 1,111 patients and found the BF rate to be 5% and 12%, respectively, in those who had RP within 6 months and those who had RP more than 6 months after the diagnosis (p=0.04).
Boorjian et al. [14] followed up their 3,149 RP cases for a mean period of 5.4 years and could not show any effect of treatment delay on BF. In the past decade, large studies performed in Johns Hopkins [8], Memorial Sloan-Kettering [15], and Sweden [16] did not show a deleterious effect of treatment delay on the oncological results.
BF is the best indicator of early PCa recurrence. Although a number of studies have studied the effect of treatment delay on BF, only a few have studied the effect of this delay on GSU. Holmstrom et al. [16] investigated the effects of primary and deferred RP on GSU, positive surgical margins, extraprostatic extension, and PCa-related death in their study with 2,566 patients. The authors found rates of GSU of 25% in primary RP and 38% in deferred RP (p<0.001).
Nowadays, the rate of GSU has been found to be 30% to 50% in a number of simultaneous RP series [17,18,19]. In our study, the GSU rate of 41.7% is similar to the results of the aforementioned studies. This means that one third of tumors are not graded correctly. These tumors in fact have a GS equal to or greater than 7 and would have lower biochemical-recurrence-free survival rates [20]. We can treat these patients better if we know the risk factors for GSU.
Davies et al. [21] investigated the effect of prostate volume on GSU in 1,251 patients and determined a larger prostate size in the group without GSU. The authors found that high PSA, greater number of cores, and a high tumor involvement rate were statistically significantly higher in the upgrade group. However, those authors stated that the duration between biopsy and surgery did not affect upgrading (p=0.2).
Authors who have studied the importance of prostate volume on GSU have reported conflicting results. The investigators who supported the finding that the rate of upgrading is higher in large prostates argued for sampling errors. They claimed that the probability of identifying previously overlooked high-grade foci postoperatively is high because it is not possible to examine a large prostate completely preoperatively. On the other hand, other investigators claimed that the possibility of high-grade cancer is higher in small prostates, and that this is why the probability of postoperative GSU is higher in those patients [17,22].
The SEARCH database study of Turley et al. [12] reported that the GSU risk might decrease in large prostates with an increase in core numbers. Those authors showed that the upgrade rate increased as the number of cores increased in prostates smaller than 30 g, whereas it decreased in prostates larger than 50 g. They also reported that GSU was related not only to the size of the prostate but also to the number of cores obtained.
Freedland et al. [23] reported that the risk of GSU was 7.5 times higher in prostates <20 mL than in those ≥100 mL. Similarly, the results of Turley et al support these findings [11].
Serkin et al. [24] found a GSU rate of approximately two times in those with a biopsy core positivity of more than 50%. Kundu et al. [25] showed that increased PSAD was associated with more aggressive clinically localized PCa. In our study, we found a higher upgrade rate in patients with low prostate volumes and high PSAD, similar to the aforementioned study (43.8 mL vs. 59.5 mL, p=0.0001). We have further evidence showing that smaller prostates with increased PSAD may be more likely to be upgraded and may harbor a cancer more aggressive than the initial biopsy revealed. The multivariate logistic regression results showed that decrease in prostate volume was a prognostic factor for GSU (odds ratio, 1.07; p<0.0001).
Hong et al. [26] investigated predictive factors for GSU risk in 203 patients in whom 12-core biopsies were obtained. The authors found similar age, body mass index, presence of hypoechoic lesions on TRUS, prostate volume, and clinical grade in the upgrade and nonupgrade groups; however, the preoperative PSA level, PSAD, number of positive cores, total biopsy tumor length, and the average tumor percentage in the core were significantly higher in the upgrade group. Similar studies have shown that high PSA and high tumor core percentage have important effects on GSU [27,28]. In concordance with the literature, positive surgical margin, extraprostatic extension, and capsular invasion were statistically significantly higher in the upgrade group (p=0.0001, p=0.0001, and p=0.001, respectively). The tumor percentage in the cores was higher in the upgrade group (p=0.001), in concordance with the literature [21,26]. In addition, logistic regression analysis showed that an increase in percentage of tumor was independently associated with 6.16 increased odds of GSU (95% confidence interval, 2.145-17.722).
Richstone et al. [29] reported that upgrade risk increased over 70 years of age when compared to age under 70 years. In another study, no relation was found between age and GSU [30]. Similarly, the ages were similar in the upgrade and nonupgrade groups in our study.
The time from biopsy to surgery was significantly longer in patients who had an upgrade (p=0.004). Time from biopsy to surgery is an independent prognostic risk factor for GSU (odds ratio, 1.023; p=0.002). These results indicate that the period between biopsy and surgery is a significant factor that affects GSU.
The limitations of our study were its retrospective design and the limited number of patients. However, the inclusion of only clinical stage T1c patients, the collection of 12-core biopsies from all patients, and a relatively narrow PSA range (PSA<20 ng/dL) increase the power of our study.
Figures and Tables
TABLE 1
Comparison of age, total PSA, prostate volume, PSAD, time from biopsy to surgery, the average core length in prostate biopsy, the average percentage of tumor in cores, and pathologic tumor volume between the groups
References
1. van den Bergh RC, Roemeling S, Roobol MJ, Roobol W, Schroder FH, Bangma CH. Prospective validation of active surveillance in prostate cancer: the PRIAS study. Eur Urol. 2007; 52:1560–1563.
2. Mohler J, Bahnson RR, Boston B, Busby JE, D'Amico A, Eastham JA, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010; 8:162–200.
3. Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007; 177:2106–2131.
4. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007; 57:43–66.
5. Danziger M, Shevchuk M, Antonescu C, Matthews GJ, Fracchia JA. Predictive accuracy of transrectal ultrasound-guided prostate biopsy: correlations to matched prostatectomy specimens. Urology. 1997; 49:863–867.
6. Nguyen PL, Whittington R, Koo S, Schultz D, Cote KB, Loffredo M, et al. The impact of a delay in initiating radiation therapy on prostate-specific antigen outcome for patients with clinically localized prostate carcinoma. Cancer. 2005; 103:2053–2059.
7. Nam RK, Jewett MA, Krahn MD, Robinette MA, Tsihlias J, Toi A, et al. Delay in surgical therapy for clinically localized prostate cancer and biochemical recurrence after radical prostatectomy. Can J Urol. 2003; 10:1891–1898.
8. Khan MA, Mangold LA, Epstein JI, Boitnott JK, Walsh PC, Partin AW. Impact of surgical delay on long-term cancer control for clinically localized prostate cancer. J Urol. 2004; 172(5 Pt 1):1835–1839.
9. Sved PD, Gomez P, Manoharan M, Kim SS, Soloway MS. Limitations of biopsy Gleason grade: implications for counseling patients with biopsy Gleason score 6 prostate cancer. J Urol. 2004; 172:98–102.
10. Stackhouse DA, Sun L, Schroeck FR, Jayachandran J, Caire AA, Acholo CO, et al. Factors predicting prostatic biopsy Gleason sum under grading. J Urol. 2009; 182:118–122.
11. Turley RS, Hamilton RJ, Terris MK, Kane CJ, Aronson WJ, Presti JC Jr, et al. Small transrectal ultrasound volume predicts clinically significant Gleason score upgrading after radical prostatectomy: results from the SEARCH database. J Urol. 2008; 179:523–527.
12. Turley RS, Terris MK, Kane CJ, Aronson WJ, Presti JC Jr, Amling CL, et al. The association between prostate size and Gleason score upgrading depends on the number of biopsy cores obtained: results from the Shared Equal Access Regional Cancer Hospital Database. BJU Int. 2008; 102:1074–1079.
13. O'Brien D, Loeb S, Carvalhal GF, McGuire BB, Kan D, Hofer MD, et al. Delay of surgery in men with low risk prostate cancer. J Urol. 2011; 185:2143–2147.
14. Boorjian SA, Bianco FJ Jr, Scardino PT, Eastham JA. Does the time from biopsy to surgery affect biochemical recurrence after radical prostatectomy? BJU Int. 2005; 96:773–776.
15. Vickers AJ, Bianco FJ Jr, Boorjian S, Scardino PT, Eastham JA. Does a delay between diagnosis and radical prostatectomy increase the risk of disease recurrence? Cancer. 2006; 106:576–580.
16. Holmstrom B, Holmberg E, Egevad L, Adolfsson J, Johansson JE, Hugosson J, et al. Outcome of primary versus deferred radical prostatectomy in the National Prostate Cancer Register of Sweden Follow-Up Study. J Urol. 2010; 184:1322–1327.
17. D'Amico AV, Renshaw AA, Arsenault L, Schultz D, Richie JP. Clinical predictors of upgrading to Gleason grade 4 or 5 disease at radical prostatectomy: potential implications for patient selection for radiation and androgen suppression therapy. Int J Radiat Oncol Biol Phys. 1999; 45:841–846.
18. Chun FK, Steuber T, Erbersdobler A, Currlin E, Walz J, Schlomm T, et al. Development and internal validation of a nomogram predicting the probability of prostate cancer Gleason sum upgrading between biopsy and radical prostatectomy pathology. Eur Urol. 2006; 49:820–826.
19. Fukagai T, Namiki T, Namiki H, Carlile RG, Shimada M, Yoshida H. Discrepancies between Gleason scores of needle biopsy and radical prostatectomy specimens. Pathol Int. 2001; 51:364–370.
20. D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998; 280:969–974.
21. Davies JD, Aghazadeh MA, Phillips S, Salem S, Chang SS, Clark PE, et al. Prostate size as a predictor of Gleason score upgrading in patients with low risk prostate cancer. J Urol. 2011; 186:2221–2227.
22. Kassouf W, Nakanishi H, Ochiai A, Babaian KN, Troncoso P, Babaian RJ. Effect of prostate volume on tumor grade in patients undergoing radical prostatectomy in the era of extended prostatic biopsies. J Urol. 2007; 178:111–114.
23. Freedland SJ, Isaacs WB, Platz EA, Terris MK, Aronson WJ, Amling CL, et al. Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: a search database study. J Clin Oncol. 2005; 23:7546–7554.
24. Serkin FB, Soderdahl DW, Cullen J, Chen Y, Hernandez J. Patient risk stratification using Gleason score concordance and upgrading among men with prostate biopsy Gleason score 6 or 7. Urol Oncol. 2010; 28:302–307.
25. Kundu SD, Roehl KA, Yu X, Antenor JA, Suarez BK, Catalona WJ. Prostate specific antigen density correlates with features of prostate cancer aggressiveness. J Urol. 2007; 177:505–509.
26. Hong SK, Han BK, Lee ST, Kim SS, Min KE, Jeong SJ, et al. Prediction of Gleason score upgrading in low-risk prostate cancers diagnosed via multi (> or = 12)-core prostate biopsy. World J Urol. 2009; 27:271–276.
27. Gofrit ON, Zorn KC, Taxy JB, Lin S, Zagaja GP, Steinberg GD, et al. Predicting the risk of patients with biopsy Gleason score 6 to harbor a higher grade cancer. J Urol. 2007; 178:1925–1928.
28. Dong F, Jones JS, Stephenson AJ, Magi-Galluzzi C, Reuther AM, Klein EA. Prostate cancer volume at biopsy predicts clinically significant upgrading. J Urol. 2008; 179:896–900.
29. Richstone L, Bianco FJ, Shah HH, Kattan MW, Eastham JA, Scardino PT, et al. Radical prostatectomy in men aged >or=70 years: effect of age on upgrading, upstaging, and the accuracy of a preoperative nomogram. BJU Int. 2008; 101:541–546.
30. Bright E, Manuel C, Goddard JC, Khan MA. Incidence and variables predicting Gleason score up-grading between trans-rectal ultrasound-guided prostate biopsies and radical prostatectomy. Urol Int. 2010; 84:180–184.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download