Abstract
We report the case of a 55-year-old woman with bilateral, large, calcified adrenal tumors who was treated by laparoscopic adrenalectomy. The patient presented with upper abdominal discomfort for the past 5 years. Her imaging showed bilateral enlarged adrenal glands up to 10-cm size with punctate calcifications. Positron emission tomography scan demonstrated moderate fluorodeoxyglucose avidity in the left adrenal mass. Bilateral laparoscopic adrenalectomy was performed through a transperitoneal approach. The postoperative period was uneventful, and the patient was discharged on the third postoperative day. Histology findings were consistent with adrenal leiomyomatosis.
Incidental adrenal masses are often discovered during investigative studies for unrelated abdominal or thoracic pathology [1]. Surgical intervention for adrenal masses is always warranted in the setting of functional tumors or when the likelihood of malignancy is high. Calcified adrenal masses are usually secondary to infections like tuberculosis, histoplasmosis, adrenal hemorrhage, or pseudocyst. Some adrenal tumors may also calcify, such as neuroblastoma or pheochromocytoma [2]. Leiomyomas of the adrenal gland are very rare [3]. They are benign in nature and composed of smooth muscle cells. Interestingly, most of these tumors have been seen in immunocompromised populations, but they do occur in normal populations as well [4,5]. Calcified adrenal leiomyomas have not been reported earlier in the literature. Laparoscopic adrenalectomy is the standard of care for most adrenal tumors, although the procedure may be technically challenging for large masses [6,7]. We report the successful laparoscopic removal of large bilateral adrenal leiomyomas.
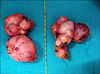
A 55-year-old woman presented with abdominal discomfort for the past 5 years. Significantly, the patient had been treated for pulmonary tuberculosis 10 years previously. She had no other comorbid illness. Plain x-ray and contrast-enhanced computed tomography (CT) of the abdomen showed bilateral, enlarged adrenal glands up to 10 cm in size with punctate calcifications (Fig. 1). The masses were found to be nonfunctional (serum electrolytes, cortisol, and urinary metanephrines were within the normal range). The results of serology for histoplasma antigen and polymerase chain reaction for mycobacterium were negative. Positron emission tomography scan demonstrated moderate fluorodeoxyglucose avidity in the left adrenal mass. Bilateral simultaneous laparoscopic adrenalectomy was performed transperitoneally (Fig. 2). The postoperative period was uneventful, and the patient was started on intravenous hydrocortisone, which was subsequently switched over to oral prednisolone. The surgical specimen was lobulated, well encapsulated, pale, and firm in consistency and measured about 10 cm×6 cm×4 cm (Fig. 3). The tumor was encapsulated and hard in consistency. The cut surface was whitish with areas of whorling and calcification. Histological examination of both masses showed well-encapsulated tumor comprising spindle cells in interlacing long and short fascicles with extensive areas of hyalinization with peripherally compressed adrenal tissue. The individual cells had oval to spindle nuclei with blunted ends and a moderate amount of eosinophilic cytoplasm. No mitosis or necrosis was identified. Areas of calcification were seen. On immunohistochemistry, the tumor cells were positive for smooth muscle actin but negative for S-100 (Fig. 4).
We used a transperitoneal approach in the lateral position, which was changed according to the side. A 12-mm umbilical camera port was inserted after creation of pneumoperitoneum, which was used for both sides. A 5-mm epigastric retraction port (common to both sides); 12-mm working ports on the corresponding sides along the anterior axillary line, midway between the epigastric and umbilical ports; and another 5-mm working port on both sides along the midclavicular line were inserted under vision. Bilateral adrenal glands were identified after mobilization of the colon. Intraoperatively, both adrenal glands were enlarged, measuring approximately 10 cm×6 cm each side, and were lobulated and well encapsulated. Planes with surrounding viscera were well preserved. Both tumors were dissected and the specimens were retrieved by using an indigenously designed EndoCatch bag. The procedure was completed in 1 hour 45 minutes. The total blood loss was 100 mL.
Adrenal calcification is an uncommon clinical entity [2]. Adrenal hemorrhage and tuberculosis are the most common causes of adrenal calcifications. Adrenal hemorrhages are most often unilateral (up to 80%) and involve the right adrenal in 85% of cases. Adrenal hemorrhage and hemorrhagic adrenal tumors may appear similar. Acute hemorrhage is hyperdense and associated with inflammatory stranding and enlarged adrenals. Calcifications may be seen within weeks of hemorrhage. Follow-up CT usually shows resolution of hemorrhage but tumors persist. Bronchogenic carcinoma metastasizing to the adrenals is the most common cause of an enlarged and hemorrhagic enhancing adrenal malignancy. Granulomatous diseases like tuberculosis and histoplasmosis can cause diffuse adrenal calcifications. When chronically infected, the adrenal glands may contain increased amounts of calcium, which can be thick and irregular. Evidence of granulomatous disease elsewhere in the body is helpful in establishing this diagnosis. When chronic granulomatous infection causes adrenal insufficiency, the adrenals will be atrophic and densely calcified. Adrenal pseudocysts are the most common cause of calcified adrenal masses in adults. Tumors that calcify include neuroblastoma and ganglioneuroma in children and adrenal carcinoma, pheochromocytoma, and ganglioneuroma in adults. Stippled or coarse calcifications are seen in 70% of neuroblastoma, which is the most common solid abdominal mass in infancy and is unilateral (90%). Pheochromocytoma can be bilateral in 10% of cases and 10% are associated with calcifications. Calcifications are rare in adrenal cortical adenomas. Myelolipomas typically present as large, fat-containing tumors, of which about 20% show calcifications. Addison's disease produces adrenal calcification in 25% of cases over a chronic course. Adrenal hemangiomas are rare benign tumors that are usually asymptomatic but may cause hemorrhage or pain if large enough to exert a mass effect on adjacent organs. Characteristically, these lesions contain phleboliths, and this finding should strongly suggest the proper diagnosis. However, if there has been previous hemorrhage, irregular or nodular calcification may also be present.
In this patient, our preoperative differential diagnosis included the possibility of a ganglioneuroma, histoplasmosis, or tuberculosis. As such, the pathological finding of an adrenal leiomyoma came as a surprise because adrenal leiomyomas presenting as calcified masses has not been described before. Although leiomyomas of the gastrointestinal tract are quite common, adrenal leiomyomas are very rare. Only a few cases have been reported in the literature. Most of these cases have involved patients who were immunocompromised. The link between the immune system and smooth muscle tumors of the adrenal gland is unclear. It has been postulated that infection with the human immunodeficiency virus (HIV) may promote smooth muscle tumors. HIV may have a direct or indirect oncogenic stimulatory effect [4,5].
Laparoscopic adrenalectomy has become the gold standard in most patients with adrenal tumors [6,7]. Laparoscopic procedures are associated with less postoperative discomfort, shorter hospital stay, and a lower rate of complications. Large but well-encapsulated adrenal masses without evidence of local invasion can be removed laparoscopically. The transperitoneal approach offers better visualization of major vessels. Several authors limit the laparoscopic adrenalectomy to lesions less than 6 cm in size, whereas some have performed laparoscopic adrenalectomy on tumors up to 13 cm. Nguyen et al. [8] reported their 10-year experience with laparoscopic adrenalectomy in 150 patients to be safe and effective. However, they also recommended referral to a specialized center for optimal outcomes. We believe that laparoscopic adrenalectomy is safe and effective in carefully selected cases. Size is a criterion, but in cases without local invasion, the laparoscopic approach can be a viable option.
In summary, differential diagnosis for adrenal calcified masses includes a heterogeneous group of conditions including infections and malignant and benign tumors. Hence, such patients should be meticulously worked up. Adrenal leiomyomas should also be hitherto considered as a differential diagnosis of such lesions. Laparoscopic resection of large tumors is feasible but necessitates experience in advanced laparoscopic surgery.
Figures and Tables
FIG. 1
Contrast-enhanced computed tomography of the abdomen showing bilateral calcified adrenal masses.
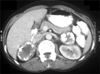
FIG. 2
Intraoperative photographs showing the left adrenal tumor being dissected. Arrows indicating adrenal tumor.
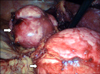
FIG. 4
(A) Normal adrenal gland seen at the periphery with encapsulated tumor arranged in long and short fascicles (H&E, ×40). (B) Higher magnification showing fascicles of benign spindle-shaped cells with minimal nuclear atypia (H&E, ×200). (C) Neoplastic cells showing strong positivity with smooth muscle actin (immunoperoxidase, ×200).

ACKNOWLEDGMENTS
We acknowledge the contributions of Dr. Ashim Das, Professor, Department of Pathology, Post Graduate Institute of Medical Education and Research, for his contribution of the histopathology slides and descriptions.
References
1. Brunt LM, Moley JF. Adrenal incidentaloma. World J Surg. 2001; 25:905–913.
2. Hindman N, Israel GM. Adrenal gland and adrenal mass calcification. Eur Radiol. 2005; 15:1163–1167.
3. Jacobs IA, Kagan SA. Adrenal leiomyoma: a case report and review of the literature. J Surg Oncol. 1998; 69:111–112.
4. Parola P, Petit N, Azzedine A, Dhiver C, Gastaut JA. Symptomatic leiomyoma of the adrenal gland in a woman with AIDS. AIDS. 1996; 10:340–341.
5. Jimenez-Heffernan JA, Hardisson D, Palacios J, Garcia-Viera M, Gamallo C, Nistal M. Adrenal gland leiomyoma in a child with acquired immunodeficiency syndrome. Pediatr Pathol Lab Med. 1995; 15:923–929.
6. Jacobs JK, Goldstein RE, Geer RJ. Laparoscopic adrenalectomy: a new standard of care. Ann Surg. 1997; 225:495–501.
7. Gagner M, Lacroix A, Bolte E, Pomp A. Laparoscopic adrenalectomy. The importance of a flank approach in the lateral decubitus position. Surg Endosc. 1994; 8:135–138.
8. Nguyen PH, Keller JE, Novitsky YW, Heniford BT, Kercher KW. Laparoscopic approach to adrenalectomy: review of perioperative outcomes in a single center. Am Surg. 2011; 77:592–596.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download