Abstract
Bladder cancer is the most prevalent malignancy of the urinary tract. About 90% of bladder cancers are urothelial carcinomas. Seventy percent of cases newly diagnosed are superficial diseases; roughly 30% of newly diagnosed cases are muscle-invasive metastatic diseases. Bladder urothelial carcinoma primarily metastasizes into regional lymph nodes and then into liver, lung, mediastinum, bone, and adrenal gland. In our case, non-muscle-invasive bladder cancer metastasized into the bone, mediastinum, iliac lymph node, and adrenal and thyroid glands. This is the first reported case in the current literature in which urothelial carcinoma metastasized into the thyroid gland.
Bladder cancer is the most prevalent malignancy of the urinary tract. Its incidence worldwide is 10.1 in every 100,000 men and 2.5 in every 100,000 women. The highest incidence rate of bladder cancer in Europe is seen in the western and southern regions, whereas Eastern Europe has the lowest incidence rate. The worldwide mortality rate is 4 in every 100,000 men and 1.1 in every 100,000 women. The mortality rate of bladder cancer in Europe in the past decade was 16% in men and 12% in women [1].
Approximately 90% of bladder cancers are urothelial carcinomas. Seventy percent of cases of newly diagnosed disease are superficial disease; roughly 30% of newly diagnosed cases are muscle-invasive metastatic diseases. Although local recurrence is significant despite treatment, the natural progression of non-muscle-invasive bladder cancer tends to remain superficial. About 5% to 20% of superficial tumors will develop into muscle-invasive disease despite treatment [2].
The primary propagation of bladder urothelial carcinomas is into the regional lymph nodes. The most common areas of distant metastases of urothelial carcinomas are typically the liver, lung, mediastinum, bone, and adrenal gland, respectively [3]. We present a case of non-muscle-invasive bladder cancer that metastasized into the bone, mediastinum, iliac lymph node, and adrenal and thyroid glands.
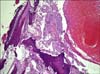
An 83-year-old male patient visited the Urology Department with complaints of macroscopic hematuria at intervals over 9 months. Transurethral resection of the bladder (TUR-B) was performed on the patient when a 17-mm×14-mm mass was found in the ultrasound examination. The pathological result was a high-grade superficial papillary urothelial carcinoma (Fig. 1). Repeat TUR was applied to the patient 4 weeks later and high-grade urothelial carcinoma with lamina propria invasion was found, whereas muscularis propria invasion did not exist. Positron emission tomography (PET) was performed during the second month because the patient had chest pain in the postoperative period, and multiple metastatic bone lesions, metastatic lymphadenopathies on the mediastinum and right external iliac area, a hypermetabolic lesion on the left thyroid lobe, and a hypermetabolic lesion on the left adrenal gland were found. Incisional biopsy performed on the fifth rib of the patient was evaluated as urothelial carcinoma metastasis and radiotherapy was applied to the patient for these lesions (Fig. 2).
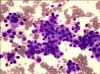
In the physical examination of the patient, a fixed, 3-cm mass was palpated on the left lobe of the thyroid. Then, in the neck computed tomography (CT) with contrast, a 40 mm×34 mm nodular mass was found on the left lobe of the thyroid gland, which caused left-sided destruction of the thyroid cartilage. The mass was evaluated as a hypoactive nodule in thyroid scintigraphy. A hypermetabolic nodular lesion was seen on the left thyroid lobe by PET. Malignant tumor cells were observed in the fine-needle aspiration sample taken from the hypermetabolic lesion on the left lobe of the thyroid. Owing to the patient's clinical history, primary thyroid carcinomas were considered in the differential diagnosis, because metastatic carcinoma and anaplastic and medullar carcinomas are of first priority. The immunohistochemical panel included thyroid transcription factor-1 (TTF-1) and thyroglobulin in terms of thyroid tumors; calcitonin, carcinoembryonic antigen (CEA), chromogranin A, and synaptophysin in terms of medullar carcinoma; and cytokeratins (CK) 7 and CK 20 antibodies in terms of urothelial carcinoma. No reaction was observed with neuroendocrine markers (synaptophysin and chromogranin), calcitonin, thyroglobulin, or TTF-1 in tumoral cells. Positivity was established in some cells by CEA and CK 20. The tumor was evaluated as a urothelial carcinoma metastasis owing to the clinical history of the patient, the existence of other metastases, the morphological similarity of the tumor cells with the primary tumor, and CK 20 positivity in some cases immunohistochemically (Fig. 3). Because the general condition of the patient worsened while planning chemotherapy, no systematic chemotherapy was applied. After 6 months, the patient died.
The prognosis and survival of patients with bladder cancer are associated with stage and grade during diagnosis. Survival rates for 5 years were reported as 94% for grade 1 non-muscle-invasive disease, 40% for grade 3 non-muscle-invasive disease, 72% for stage T2, and 33% for stage T4 muscle-invasive disease [4].
Contrary to muscle-invasive disease, many superficial bladder cancers can be treated by regular monitoring cystoscopy, urinary cytology, and re-TUR together or not with protective therapy, transurethral resection, and adjuvant intravesical treatment. Despite these measures, about 5% to 20% of non-muscle-invasive tumors will progress to muscle-invasive disease. High-grade non-muscle-invasive tumors are related to a high risk of muscular invasion and metastasis and end up with a worse prognosis [5]. Also, it is known that metastasis can appear without muscular invasion; these metastases are found in regional lymph nodes during cystectomy [5]. Cases of distant metastasis of low-grade non-muscle-invasive urothelial carcinoma without regional lymph node metastasis have been reported [6]. Errors in clinical staging of bladder tumors are well known. In some pathological staging cases, it is estimated that low staging was done at a rate as high as 30% to 40% [7].
The European Organisation for Research and Treatment of Cancer (EORTC) developed a scoring system and a risk table to estimate short- and long-term risks of recurrence and progression separately. In this scoring system, clinical and pathological parameters are tumor number, tumor size, previous recurrence rate, T phase, carcinoma in situ presence, and tumor grade [8]. In our patients, the recurrence rate was found to be 6 and the progression score was 12. Possible mean progression rates according to the score are reported as 5% in the first year and 17% in the fifth year.
In the EORTC study, no results are reported for new concepts such as re-TUR, early single-dose chemotherapy, and maintenance bacillus Calmette Guerin treatment, and this has been criticized by authors [8]. In our patient, early single-dose chemotherapy and re-TUR were applied and both pathology reports suggested a high-grade non-muscle-invasive bladder tumor. Although the progression rate of our patient for 1 year was estimated as 5% according to the EORTC study, progression developed in the patient before initiating induction treatment of intravesical CT and before symptoms associated with bone metastases developed.
There are publications in the current literature that report that low-grade non-muscle-invasive bladder tumors are detected clinically and the distant lung metastasis can appear without regional lymph node involvement. Rare metastases of urothelial carcinoma into the skin, uterus, and orbita and vaginal and omental involvements have also been reported [3].
Puente et al. [9] showed a small cell carcinoma that metastasized into the thyroid gland, which is a minor variant of bladder urothelial carcinoma. Small-cell carcinoma is rarely originated from the bladder and comprises of 0.48% to 1% of all bladder tumors. Metastases in the thyroid gland are observed in many patients by physical examination or imaging methods during routine monitoring after primary tumor resection. In these patients, fine-needle aspiration may be useful for evaluating possibilities and limiting unnecessary operation for diagnostic purposes.
The most significant conflict in cytologic comment is the differentiation between primary thyroid anaplastic carcinoma and high-grade metastatic malignity. In the fine-needle aspiration material of the patient, differential thyroid carcinomas and medullar carcinomas can be excluded morphologically and immunohistochemically. In the present case, no reaction was observed with neuroendocrine markers (synaptophysin and chromogranin A), calcitonin, thyroglobulin, or TTF-1 in tumoral cells. Positivity was established in some cells by CEA and CK 20.
Although a positive reaction immunohistochemically with thyroglobulin supports a primary thyroid malignancy, only 20% to 30% of anaplastic carcinomas display thyroglobulin positivity and negative results do not have meaning. Because 20% to 30% of anaplastic carcinomas display thyroglobulin positivity and negative results do not have meaning, TTF-1 and thyroglobulin negativity does not exclude the possibility of anaplastic carcinoma. Urothelial carcinomas display positivity with CK 7 and CK 20 antibodies. The patient's clinical history is the most essential part in the differential diagnosis of these lesions, and it is emphasized that a thyroid nodule found in a patient with a known cancer should be accepted as metastasis unless proven otherwise [10]. Because our patient had a known urothelial carcinoma history, other metastases, a morphological similarity between the tumor cells and the primary tumor, and CK 20 positivity immunohistochemically in some cells, the tumor was evaluated as a urothelial carcinoma metastasis.
In conclusion, non-muscle-invasive bladder tumors may present with multiple distant metastases even without muscle invasion. This presented case showed that bladder tumors can metastasize into the thyroid gland in addition to known distant metastasis areas. Metastasis of a bladder tumor should also be kept in mind in the differential diagnosis in patients who have a bladder tumor history and are found to have a mass on the thyroid gland.
Figures and Tables
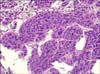
FIG. 1
Bladder transurethral resection biopsy: high-grade urothelial carcinoma with invasion of the lamina propria by malignant epithelial tumor cells with large, pleomorphic nuclei (H&E, ×200).
References
1. Ferlay J, Randi G, Bosetti C, Levi F, Negri E, Boyle P, et al. Declining mortality from bladder cancer in Europe. BJU Int. 2008; 101:11–19.
2. Dougherty DW, Gonsorcik VK, Harpster LE, Trussell JC, Drabick JJ. Superficial bladder cancer metastatic to the lungs: two case reports and review of the literature. Urology. 2009; 73:210.e3–210.e5.
3. Alexander PW, Sanders C, Nath H. Cavitary pulmonary metastases in transitional cell carcinoma of urinary bladder. AJR Am J Roentgenol. 1990; 154:493–494.
4. Nishiyama H, Habuchi T, Watanabe J, Teramukai S, Tada H, Ono Y, et al. Clinical outcome of a large-scale multi-institutional retrospective study for locally advanced bladder cancer: a survey including 1131 patients treated during 1990-2000 in Japan. Eur Urol. 2004; 45:176–181.
5. Gwynn ES, Clark PE. Bladder cancer. Curr Opin Oncol. 2006; 18:277–283.
6. Kardar AH, Lindstedt EM, Tulbah AM, Bazarbashi SN, al Suhaibani HS. Metastatic transitional cell carcinoma of the ovary from superficial bladder tumour. Scand J Urol Nephrol. 1998; 32:73–76.
7. Dutta SC, Smith JA Jr, Shappell SB, Coffey CS, Chang SS, Cookson MS. Clinical under staging of high risk nonmuscle invasive urothelial carcinoma treated with radical cystectomy. J Urol. 2001; 166:490–493.
8. Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Bohle A, Palou-Redorta J, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011; 59:997–1008.
9. Puente S, Velasco A, Gallel P, Pallares J, Perez-Ruiz L, Ros S, et al. Metastatic small cell carcinoma to the thyroid gland: a pathologic and molecular study demonstrating the origin in the urinary bladder. Endocr Pathol. 2008; 19:190–196.
10. Schröder S, Wodzynski A, Padberg B. Cytokeratin expression of benign and malignant epithelial thyroid gland tumors: an immunohistologic study of 154 neoplasms using 8 different monoclonal cytokeratin antibodies. Pathologe. 1996; 17:425–432.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download