Abstract
Spermatocytic seminoma (SCS) with sarcoma is an extremely rare testicular tumor with only 11 cases previously described in the literature. We present the 12th case of SCS with sarcoma in a 29-year-old male. SCS itself is an uncommon germ cell tumor with a relatively indolent clinical course that mostly affects males around the fifth decade of life. Sarcomatous differentiation of SCS occurs in 5% to 6% of cases and correlates with a higher possibility of metastatic disease and a poor prognosis. Clinically, this tumor manifests as a slow-growing testicular mass often with an accelerated period of secondary growth. After a concise review of the literature, we conclude that SCS with sarcoma should be treated by radical inguinal orchidectomy with strong consideration given to adjuvant chemotherapy.
Spermatocytic seminoma (SCS) is an uncommon germ cell tumor (GCT). It accounts for 4% to 7% of all cases of testicular seminoma [1]. The mean age of diagnosis of SCS is 54 years [2]. Clinically, SCS is an indolent neoplasm with rare potential to metastasize and therefore is associated with a good prognosis [3]. Sarcomatous transformation of GCTs is associated with a significant propensity for aggressive and malignant tumor behavior [4]. Specifically, the development of a sarcomatous component in testicular GCT is described as an uncommon phenomenon [4]. The development of a sarcomatous component in SCS is extremely rare, and only a handful of cases have been described in the literature (Table 1). We present an exceptionally rare case of SCS with undifferentiated sarcomatous transformation in a young male. We believe this to be only the 12th report to date of SCS with sarcomatous differentiation.
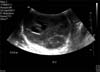
A 29-year-old male of Syrian origin presented with a painless right-sided testicular swelling of 12 months' duration. Clinical examination revealed a nontender enlarged right testis. The tumor markers alpha-fetoprotein (α-FP) and beta human chorionic gonadotropin (β-HCG) were negative. An ultrasound scan of the scrotum revealed a 7-cm hypoechoic, hypervascular and cystic mass replacing the right testis (Fig. 1). The patient underwent a right radical inguinal orchidectomy. A staging computed tomography scan was negative for lymphatic or metastatic disease.
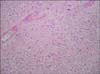
Macroscopically, a lobulated, circumscribed, focally necrotic, soft tumor (60 mm×55 mm×50 mm) was found replacing most of the right testis. The tumor appeared to be confined within the testis. The majority of the tumor was polymorphous and was composed of small, intermediate, and large epithelioid cells arranged in sheets with broad areas of necrosis. Microscopic features of these cells were consistent with SCS. Additionally, there were ill-defined areas of undifferentiated sarcoma (Figs. 2, 3) that were composed of highly pleomorphic, epithelioid, and spindle-shaped cells with high mitotic activity invested in fibrous and fibromyxoid stroma. The tumor did not extend beyond the tunica albuginea. The spermatic cord and epididymis were uninvolved. No lymphovascular invasion was identified. Immunohistochemical analysis revealed focal staining of a small proportion of the SCS cells with antibody to CD117 (c-KIT). Immunohistochemical stains of antibodies to PLAP, HCG, AFP, CD30, and CD68 and the myeloid markers CD34 and CD117 were all negative. The histological diagnosis was SCS with undifferentiated sarcoma confined to the testis. Further oncological treatment with adjuvant chemotherapy was strongly advised but the patient refused and moved to a different locality.
Testicular seminoma accounts for 40% to 50% of all GCTs of the testis [5], with SCS accounting for less than 1% of all testicular tumors [6]. SCS occurs purely in the testis and in an older age group (mean age, 54 years; range, 25 to 87 years) compared with classic seminoma (mean age, 41 years; range, 30 to 40 years) [1,2,7]. Diagnosis of SCS is based on histopathological features including the size spectrum of cells, lack of cytoplasmic glycogen, and lymphocytic infiltrate [8]. Absence of granulomas and spherical, dark nuclei help to distinguish these tumors from classic seminomas [1,8]. Importantly, SCSs are not associated with concurrent germ cell components, unlike a third of classic seminomas with teratomatous-type association [1]. The clinical manifestation of SCS is classically described as a painlessly enlarging testicular mass [8]. Metastasis of SCS is extremely rare and has only been seen in 1 or 2 cases [8], leading to a favorable prognosis after orchidectomy, which is considered the gold standard treatment.
SCS with sarcoma is an extremely rare diagnosis with only 11 cases previously reported in the literature (Table 1) and with this article illustrating the 12th case. This sarcomatous differentiation occurs in approximately 5% to 6% of SCS [2,8] and is associated with aggressive behavior and poor prognosis [8]. In the few reported cases of SCS with a sarcomatous component, the mean patient age was 51 years (range, 40 to 66 years), which is similar to the age associated with pure SCS. In most of the cases outlined in Table 1, there was a dual component associated with the clinical history. First, the slow progression of a painless unilateral scrotal mass (mean, 4 years from 7 cases) is followed by a secondary accelerated period of growth, which is often painful (mean, 2 months from 6 cases). The second component of rapid growth has been suggested to be the latent sarcomatous differentiation of a pure SCS [1]. The most common sarcomatous differentiation was rhabdomyosarcoma, followed by undifferentiated and spindle cell sarcoma (Table 1). No teratomatous component was identified in any of the cases [10]. In most cases, serum tumor markers such as α-FP, β-HCG, and lactate dehydrogenase; were normal. Average tumor size was 14 cm, with most tumors being treated by radical orchidectomy and adjuvant chemotherapy. Metastatic disease at the time of presentation or histological diagnosis was present in six cases, with two cases developing metastatic disease months or years after orchidectomy and two cases without any evidence of metastatic disease at any stage. One case described by Menon et al. [2] lacked details on any metastatic disease but mentioned further care at an oncology unit. Poor prognosis of these cases is evident by mortality in seven cases with a mean survival of 7 months. In one case reported by True et al. [1], the patient developed metastases 16 years after the diagnosis, which was assumed to be a result of metastatic prostate carcinoma.
The diagnosis of SCS is rare below the age of 40 years [9] and our case of SCS with undifferentiated sarcoma in a 29-year-old man is the only case below this age reported in the literature. Our case classically reported a long duration (12 months) of painless testicular swelling. No period of painful accelerated growth was reported. The size of the tumor in this case was considerably smaller at 6 cm compared with the average 14 cm calculated from previous cases. Although SCS with sarcoma is associated with a poor prognosis and metastasis at diagnosis is seen in half of cases, our case had no evidence of extratesticular spread.
Previous articles have estimated up to 16 case reports in the literature [10]. Our literature review revealed 11 definite cases of SCS with sarcomatous components. We believe that overestimation of the number of cases has arisen because of the duplication of some reported cases (case 7 in Table 1) as explained by Matoska and Talerman [8]. Case number 11 (Table 1), one of the five first cases described by True et al. [1], was found to have extensive metastasis at presentation and histological diagnosis was made at autopsy. The highly aggressive nature of sarcomatous differentiation together with the assumed late presentation (patient age, 60 years; tumor size, 25 cm) accounts for the absence of surgical or oncological management in this case. In case 7, the method of orchidectomy was via a scrotal approach rather than the conventional, and now standard, inguinal approach. This variation in surgical technique was questioned by Floyd et al. [3] and a hemiscrotectomy and retroperitoneal lymph node dissection was performed. Rhabdomyosarcoma appears to be associated with metastatic disease in every case: 1, 2, 3, 7, and 9 [10]. Owing to the limited number of cases, no definitive conclusions can be drawn about the association of specific differentiation of sarcomatous elements and the likelihood of metastatic disease. The general consensus is that SCS with sarcoma, of any differentiation or undifferentiated, is associated with a high probability of early metastases. The mechanism of transformation is unknown but one hypothesis is anaplastic conversion or transformation of SCS [9]. The rapid acceleration growth phase demonstrated clinically is likely to represent this period of transition and histological differentiation into sarcoma [9]. Most articles suggest that in the presence of sarcomatous differentiation in SCS, adjuvant chemotherapy following orchidectomy is the choice of treatment for these highly malignant tumors [2,7,9].
In conclusion, SCS with sarcomatous differentiation is an exceptionally rare tumor with only 12 cases reported in the literature. The development of this sarcomatous element transforms the prognosis of the relatively indolent SCS tumor to a highly aggressive malignant neoplasm. These tumors often present with concurrent metastatic disease and thus treatment with adjuvant chemotherapy following radical inguinal orchidectomy should be considered in all cases.
Figures and Tables
 | FIG. 3Microscopic demonstration of the interface between the sarcoma (top right) and seminoma (bottom left) elements (×40). |
References
1. True LD, Otis CN, Delprado W, Scully RE, Rosai J. Spermatocytic seminoma of testis with sarcomatous transformation: a report of five cases. Am J Surg Pathol. 1988; 12:75–82.
2. Menon S, Karpate A, Desai S. Spermatocytic seminoma with rhabdomyosarcomatous differentiation: a case report with a review of the literature. J Cancer Res Ther. 2009; 5:213–215.
3. Floyd C, Ayala AG, Logothetis CJ, Silva EG. Spermatocytic seminoma with associated sarcoma of the testis. Cancer. 1988; 61:409–414.
4. Malagon HD, Valdez AM, Moran CA, Suster S. Germ cell tumors with sarcomatous components: a clinicopathologic and immunohistochemical study of 46 cases. Am J Surg Pathol. 2007; 31:1356–1362.
5. Henley JD, Ulbright TM. Seminoma: a review with emphasis on morphologic patterns and differential diagnosis. Pathol Case Rev. 2005; 10:167–175.
6. Robinson A, Bainbridge T, Kollmannsberger C. A spermatocytic seminoma with rhabdomyosarcoma transformation and extensive metastases. Am J Clin Oncol. 2007; 30:440–441.
7. Eble JN. Spermatocytic seminoma. Pathol Case Rev. 2005; 10:176–180.
8. Matoska J, Talerman A. Spermatocytic seminoma associated with rhabdomyosarcoma. Am J Clin Pathol. 1990; 94:89–95.
9. Trivedi P, Pasricha S, Gupta A. Spermatocytic seminoma associated with undifferentiated sarcoma: a rare case report. Indian J Pathol Microbiol. 2011; 54:138–140.
10. Chelly I, Mekni A, Gargouri MM, Bellil K, Zitouna M, Horchani A, et al. Spermatocytic seminoma with rhabdomyosarcomatous contingent. Prog Urol. 2006; 16:218–220.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download