Abstract
Purpose
The aim of this research was to evaluate the efficacy of the cystoscopic extraction and external drainage techniques for unsuccessful antegrade stenting in transplanted severe ureteral obstruction.
Materials and Methods
A total of 26 patients with severe transplanted ureteral obstruction in whom the cystoscopic extraction technique and/or external drainage technique was performed were retrospectively evaluated. After the severe obstruction was successfully traversed, balloon dilatation followed by double-J stent insertion was performed.
Results
Of the 26 patients (male:female, 9:4; mean age, 38.1 years) who underwent failed ureteral stenting with the conventional procedure, 16 patients underwent successful stenting with the cystoscopic extraction technique, and 10 patients underwent successful stenting following external drainage. The mean serum creatinine of the 26 patients before stenting was 42.9 mg/dL (range, 32.7 to 54.1 mg/dL), which decreased to 10.3 mg/dL (range, 8.7 to 11.8 mg/dL) after stenting. The complications of the procedure were lower abdominal pain in 22 patients and gross hematuria in 9 patients. All complications were relieved with medical care within 3 to 5 days after the procedure. No major complications occurred.
Late ureteric stricture or obstruction is not rare in kidney transplantation and occurs at a frequency of 2% to 7.5% in renal transplant recipients [1,2]. The use of percutaneous nephrostomy (PCN) and antegrade ureteric stent implantation to treat renal allograft stricture or obstruction seems to be safe and effective. However, it is not always possible to pass a guidewire through a tight ureteric stricture when the caliber of the ureter is too small. In the present study, we introduced an alternative technique of cystoscopic extraction and external drainage for unsuccessful antegrade percutaneous ureteric stenting in cases of severe transplanted ureteral obstruction.
We performed a retrospective review of all patients with ureteric stricture after kidney transplantation who underwent PCN and antegrade ureteric stenting in two hospitals between April 2001 and June 2011. Two hundred twenty-seven patients were treated during this interval and all patients had previously consented to the use of their medical records for research purposes. Obstruction was defined by the presence of both an increasing serum creatinine concentration and hydronephrosis on ultrasound in all patients.
During the ureteric stenting in cases of transplanted ureteric stricture or obstruction after ureterovesical anastomosis, two alternative techniques were performed instead of the conventional technique in 227 patients as below:
A cystoscopic extraction technique was preferred when the portion of the guidewire passing through the stricture into the bladder was too short for the catheter to traverse the stricture via the guidewire under C-arm fluoroscopy. We covered the renal introducer sheath with a sterile drape and changed the patient's position from the supine to the lithotomic position. Then the distal portion of the guidewire (0.035 in, Terumo, Tokyo, Japan) inserted from the renal sheath was captured by use of a grasper through a flexible cystoscope (Olympus Co., Tokyo, Japan) with the cooperation of a urologist [3]. The guidewire was extracted smoothly until its distal portion was long enough to coil within the bladder. Coiling the guidewire can provide sufficient support for the catheter to follow. As described in a previous report [4], a 5-Fr angiography catheter (Cobra angiographic catheter, Terumo, Tokyo, Japan; RUC advantage catheter, Cook, Bloomington, IN, USA) was used to pass through the pelvis and ureter until through the stricture to advance to the bladder. After the guidewire was extracted, contrast agent was injected through the catheter to ensure the location of its distal tip. A stiff guidewire (0.035 inch, Terumo) was inserted through the catheter and the distal portion was left coiled within the bladder. A balloon catheter (Admiral Xtreme, Invatec S.P.A. Roncadelle, Italy) for ureteroplasty was successfully inserted and placed centrally on the point of the stricture. Then the balloon catheter insufflated with a mixture of normal saline and contrast agent was inflated repeatedly until the balloon notch disappeared. Balloon pressure did not exceed 14 bars. Subsequently, the introducer sheath was extracted and a temporary double-J stent (Visiostar Standard, Bad Aibling, Germany) with a diameter of 6 or 7 Fr was inserted into the correct position with the proximal J within the renal pelvis and the distal J within the urinary bladder (Fig. 1).
The external drainage technique was performed to rescue the inability to cross the severe obstruction. Following the successful PCN, we performed an exchange to insert a dwelling external drainage catheter (Terumo) via a stiff guidewire, with its distal portion within the renal pelvis. Then, 10-mL urine was removed through the drainage catheter and 5-mL contrast agent was injected to ensure the location. The drainage catheter was connected to a drainage bag. With antibiotic prophylaxis, the drainage began. During the drainage, we observed the urine status and ensured that the catheter was not blocked. The urine gradually turned from turbid to clear. The drains were discontinued until we were ready to perform the subsequent balloon angioplasty and stent insertion (Fig. 2).
Preoperative investigations regarding postprocedure outcomes included renal function tests, urine culture, ultrasonography, and pyelography. Repeated attempts to pass the guidewire through the tight ureteric stricture were made with local anesthesia under fluoroscopic guidance.
The double-J stents were maintained for at least 8 weeks and were removed as the hydronephrosis progressively improved during serial ultrasonographic follow-up. Subsequently, patients were followed up for 6 months. Technical success was defined as good position of the ureteric stent with adequate internal drainage of urine with relief of hydronephrosis.
A total of 201 of 227 patients underwent successful conventional ureteric stenting procedure. The conventional procedure resulted in failure in 26 of 227 patients. Of the 26 patients, 16 patients (male:female, 11:5; mean age, 37.2 years) underwent successful stent insertion combined with the cystoscopic extraction technique. Ten patients (male: female, 7:3; mean age, 39.5 years) underwent successful stent insertion after external drainage (mean time, 56.3 hours) combined with the conventional or cystoscopic extraction technique. The mean serum creatinine before stenting was 42.9 mg/dL (range, 32.7 to 54.1 mg/dL), which decreased to 10.3 mg/dL (range, 8.7 to 11.8 mg/dL) after stenting. The complications of the procedure were lower abdominal pain in 22 patients and gross hematuria in 9 patients. The results of routine blood tests showed no decrease in hemoglobin or red blood cell count. All complications were relieved with medical care within 3 to 5 days after the procedure. No major complications were encountered during the external drainage and the following procedure. The double-J ureteric stents were removed 8 to 11 weeks after insertion. In all cases, the patients were free of urologic symptoms and signs during the 6-month follow-up after stent removal.
The favorable results and acceptance of antegrade PCN and balloon dilatation combined with ureteric stent insertion have been well documented in recent years. However, it can be most challenging for interventional radiologists or urologists to pass conventional guidewires through tight distal ureteric strictures [5,6]. To successfully traverse the ureteric stricture, a variety of modifications and innovations have been proposed in the literature. Keeling and Lee [7] described the use of microcatheters and microwires to traverse these tight ureteric strictures. In that case, a hockey stick catheter was advanced over the microcatheter/guidewire combination into the bladder. Then a stiff guidewire was exchanged via the hockey catheter, followed by balloon dilation and stent implantation. Kim and Park [3] introduced the "pullthrough" technique. In their method, a goose neck snare was introduced through a 7-Fr sheath that was inserted from the urethra. The distal tip of the stiff guidewire was captured and retrieved through the urethral introducer sheath. Then, both ends of the guidewire were secured tightly and the double-J stent was inserted. Govender et al. [8] reported that the reverse end of a 0.014-inch Dasher steerable or similar guidewire could be used to pass a tight ureteric stricture, followed by cardiac angioplasty balloon dilation. Learning from their experience, we attempted to perform a cystoscopic extraction technique when the portion of the guidewire traversing the obstruction into the bladder was too short to provide enough support for the catheter to follow it. The distal tip of the guidewire in the bladder was captured through a flexible cystoscope with the cooperation of a urologist and extracted smoothly until its distal portion coiled within the bladder. The coiled guidewire can provide sufficient support for the following steps to be performed. The 16 patients all underwent satisfactory balloon dilation and stent insertion by use of this technique. Compared with the pull-through technique, the merit of the cystoscopic extraction technique is that fewer disposable interventional materials are consumed, which reduces the cost; furthermore, extraction through the cystoscope is intuitive and clear. The shortcoming is that we need the cooperation of a urologist.
All of the above techniques focused on a catheter or stent or microguidewire "traversing" the stricture. However, in some cases, neither a hydrophilic-coated guidewire nor a microguidewire can traverse the tortuosity of the ureteric stricture because the stricture caliber is too small. Kamiyama et al. [9] reported that severe preoperative hydronephrosis was a risk factor causing stent failure. The causes of late stenosis are still poorly understood. Ischemia is probably the main reason, and ureteral devascularization results in stenosis. The donor age, the presence of delayed graft function, a stentless anastomotic technique, and more than two graft arteries all increase the risk of stenosis. These factors all produce deterioration in the distal ureteral vascularization [10,11]. Ureteric stricture regions are not only with anatomic stricture, but also accompany edematous changes of the mucosa next to hydronephrosis and hydroureter. Ureteral edema can further decrease both the local arterial blood supply and venous drainage, which lead to the development of ureteral ischemia as well as narrowing. Edema of the mucosa makes it more difficult for the guidewire to pass through the stricture. Furthermore, edema may increase the possibility of causing a perforation in the ureter while the guidewire traverses the stricture. Therefore, we understood that edema can play an important role in difficulties passing through a ureteric stricture, and that decompression can relieve the edema of the mucosa. In present study, the ultrasonography and intravenous urography showed that all 10 patients suffered with hydronephrosis and hydroureter. Depending on their circumstances, we performed repeat procedures in two steps. In the first step, we performed external drainage to treat stenosis partly caused by edema through decompression to improve the local blood supply and venous drainage. We then used a hydrophilic-coated guidewire to traverse the tight stricture after the relief of mucosal edema around the stricture site, followed by balloon dilation and stent implantation. Also, in two cases, we performed a cystoscopic extraction technique owing to the portion of the guidewire passing the stricture into the bladder being too short. We think a possible cause was residual mucosal edema and severe stricture.
The limitations of the current study are that it was retrospective in nature, and the sample size was relatively small. Furthermore, owing to the small sample size, whether the cystoscopic extraction technique was performed could not be predicted. It is known that transplanted ureteric strictures have a frequent recurrence and balloon dilatation is less effective than other techniques. Although we successfully performed stent implantation in all 26 cases, which showed an excellent long-term result, the surgical techniques of ureterovesical anastomosis should be retrospectively analyzed. However, to the best of our knowledge, this study represents the first report of the technique of the combination of a flexible cystoscopic extraction and the use of internal double J stents to rescue unsuccessful antegrade stenting in cases of severe transplanted ureteric obstruction and the first observation of external drainage duration.
We believe that the technique of cystoscopic extraction and indwelling external drainage is secure and useful for traversing a severe transplanted ureteral obstruction following initial failed stent implantation. Whether cystoscopic extraction or the external drainage technique is preferred depends on the ability of the guidewire to traverse the obstruction.
Figures and Tables
FIG. 1
Medical imaging of digital subtraction angiography: a 58-year-old patient with a transplanted ureteric obstruction. (A) The portion of the guidewire crossing the stricture into the bladder was too short for the catheter to follow it. (B) After the cystoscopic extraction technique, the distal portion of the guidewire was coiled in the bladder following balloon insertion. (C) The notch of the balloon catheter for ureteroplasty showed ureteric stricture. (D) The double-J stent was inserted successfully.
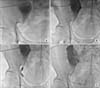
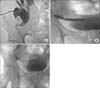
FIG. 2
Medical imaging of digital subtraction angiography: a 42-year-old patient with transplanted ureteric obstruction. (A) An external drainage catheter was inserted via the stiff guidewire with the distal portion coiled and located in the renal pelvis. (B) After external drainage for 46 hours, we successfully traversed the obstruction with a con ventional guidewire and exchanged it for a stiff guidewire. The balloon catheter was passed through the guidewire after ureteroplasty. (C) A double-J stent was inserted after balloon ureteroplasty.
References
1. Faenza A, Nardo B, Catena F, Scolari MP, d'Arcangelo GL, Buscaroli A, et al. Ureteral stenosis after kidney transplantation: a study on 869 consecutive transplants. Transpl Int. 1999; 12:334–340.
2. Hobart MG, Streem SB, Gill IS. Renal transplant complications: minimally invasive management. Urol Clin North Am. 2000; 27:787–798.
3. Kim BM, Park SI. Placement of double-J ureteric stent using the pull-through technique in patients with tight ureteric stenosis. Abdom Imaging. 2008; 33:237–240.
4. Hausegger KA, Portugaller HR. Percutaneous nephrostomy and antegrade ureteral stenting: technique-indications-complications. Eur Radiol. 2006; 16:2016–2030.
5. Atar E, Bachar GN, Bartal G, Mor E, Neyman H, Graif F, et al. Use of peripheral cutting balloon in the management of resistant benign ureteral and biliary strictures. J Vasc Interv Radiol. 2005; 16(2 Pt 1):241–245.
6. Atar E, Bachar GN, Eitan M, Graif F, Neyman H, Belenky A. Peripheral cutting balloon in the management of resistant benign ureteral and biliary strictures: long-term results. Diagn Interv Radiol. 2007; 13:39–41.
7. Keeling AN, Lee MJ. Crossing ureteric strictures: microcatheters to the rescue when conventional methods fail. Cardiovasc Intervent Radiol. 2007; 30:1234–1237.
8. Govender P, Delaney H, Guiney M. Traversing tight ureteric strictures. Cardiovasc Intervent Radiol. 2009; 32:199–201.
9. Kamiyama Y, Matsuura S, Kato M, Abe Y, Takyu S, Yoshikawa K, et al. Stent failure in the management of malignant extrinsic ureteral obstruction: risk factors. Int J Urol. 2011; 18:379–382.
10. Karam G, Hetet JF, Maillet F, Rigaud J, Hourmant M, Soulillou JP, et al. Late ureteral stenosis following renal transplantation: risk factors and impact on patient and graft survival. Am J Transplant. 2006; 6:352–356.
11. Giessing M. Transplant ureter stricture following renal transplantation: surgical options. Transplant Proc. 2011; 43:383–386.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download