Abstract
Purpose
To report our early experience with thermo-expandable urethral stents (Memokath) for the management of recurrent urethral stricture and to assess the efficacy of urethral stents.
Materials and Methods
Between March 2012 and February 2013, 13 patients with recurrent urethral stricture after several attempts with direct visual internal urethrotomy (DVIU) or failed urethroplasty underwent DVIU with thermally expandable, nickel-titanium alloy urethral stent (Memokath) insertion. Follow-up study time points were at 1, 3, 6, 9, and 12 months after stent insertion. Follow-up evaluation included uroflowmetry, retrograde urethrogram, plain radiography, and urinalysis.
Results
The mean patient age was 47.7 years (range, 18 to 74 years). The mean urethral stricture length was 5.54 cm (range, 1 to 12 cm). There were six patients with bulbar, four patients with proximal penile, one patient with distal penile, and two patients with whole penile urethral strictures, respectively. The overall success rate was 69% (9/13) and the mean postoperative peak flow rate was 17.7 mL/s (range, 6 to 28 mL/s). Major complications occurred in four patients including one patient (7.7%) with urethrocutaneous fistula induced by the stent and three patients with urethral hyperplasia. The mean follow-up duration was 8.4 months.
The natural progress of urethral stricture is poorly understood and has been sparsely reported in published studies [1]. The cause of urethral stricture can be classified as idiopathic, iatrogenic, inflammatory, and traumatic [2]. In the past, gonorrheal infection was a common cause of urethral stricture, but today in developed countries, postinflammatory stricture is rare. Meanwhile, iatrogenic causes such as transurethral endoscopic surgery, urethral catheterization, cystoscopy, prostatectomy, brachytherapy, and hypospadias surgery account for about half of the cases of urethral stricture disease treated with urethroplasty. Pelvic fracture and saddle injury are the main causes of posterior urethral stricture and are important causes in young patients [3].
Urethral stricture results from a scarring process with subsequent spongiofibrosis that is gradually progressive and results in a decrease in the diameter of the urethral lumen. Patients with urethral stricture usually complain of obstructive voiding symptoms [4,5]. Urethral stricture can cause lower urinary tract symptoms, urinary tract infection, and even renal function deterioration.
The treatment of urethral stricture includes urethral dilatation, internal urethrotomy, urethral stent placement, and open reconstruction (urethroplasty) according to the cause, site, and severity of the urethral stricture. Urethral dilatation is a simple treatment of urethral stricture and is curative only for some patients with very short and uncomplicated strictures. Internal urethrotomy is any procedure that dilates the stricture by incising or ablating it transurethrally. However, both urethral dilatation and internal urethrotomy have a high failure rate [6-9]. Although urethroplasty remains the best option with a higher success rate and a satisfactory outcome, urethroplasty has not been widely performed because it is a more invasive and technically complex procedure [10,11]. Up to now, most patients underwent multiple urethral dilatations or urethrotomies [12].
Urethral stent insertion can be another option that opposes the forces of wound contraction after direct internal urethrotomy or dilatation. Recently, a thermo-expandable stent made of nitinol (Memokath, Pnn Medical, Kvistgaard, Denmark) has been assessed in several studies as a temporary treatment for urethral strictures (Figs. 1, 2) [13]. This stent is made of a nickel-titanium alloy that has a "shape memory" feature. It is present in two crystalline forms: the more rigid form and the more pliable form. The rigid form holds the memorized shape of the Memokath at body temperature and higher. The pliable form is transformed when the alloy is cooled to ≤10℃. Most of the Memokath studies have been limited to bulbar urethral strictures, and no studies have yet been published in Korea. Accordingly, in the present study, we assessed the efficacy of thermo-expandable urethral stents (Memokath) for the management of recurrent urethral stricture and report our early experience.
All patients provided informed consent before undergoing the procedures. The case notes of 13 male patients with Memokath stent insertion for the management of recurrent urethral stricture between March 2012 and February 2013 were retrospectively reviewed. The mean symptom duration of urethral stricture was 7.57 years (range, 0.5 to 30 years). All patients had undergone direct visual internal urethrotomy or urethral dilatation or urethroplasty before the decision to perform Memokath insertion. Every patient underwent a general examination, a history taking, preoperative laboratory tests, urinalysis, retrograde urethrography (RGU), and uroflowmetry.
To perform the procedure, the surgeon should have available a cystoscopic set, urethral dilators, a guide wire, a 50-mL plastic syringe, a thermometer, two markers, and a ruler. An antibiotic was administered intravenously before the procedure. All patients received spinal anesthesia and had intraurethral 2% lidocaine gel instillation 5 minutes before the procedure. The patients were placed in the lithotomy position and draped appropriately. Cystoscopy was used to assess the site of the stricture. The urethral strictures were managed by internal urethrotomy with or without dilatation to a minimum diameter of 26 Fr. Then the length of the stricture was assessed to define the appropriate length of the Memokath stent. The cystoscopic end was placed in the proximal part of the urethral stricture. A mark was then placed on the sheath. The cystoscope was then withdrawn to the level of the distal urethral stricture and a second mark was placed at this point. A Memokath stent 10 mm longer than the measured length was chosen. If the stricture site included the bulbar urethra, a unilateral (proximal) expansion type stent was inserted. If the stricture site was limited in the penile urethra, a bilateral expansion type stent was inserted. Two stents were inserted if the length of stricture was longer than 9 cm because the maximal stent length is 9 cm. The stent was supplied on a premounted sheath. When the stent was in a satisfactory position, 150 mL of normal saline, prewarmed to 60℃, was instilled through the stent, causing expansion within the urethra. Because we have to prevent urinary retention owing to spinal anesthesia, we inserted a guide wire, and a 10-Fr nephrostomy catheter was inserted along the guide wire. The day after surgery, we removed the nephrostomy catheter. An antibiotic was administered intravenously for 2 days after the surgery, and then oral antibiotics were administered for 7 days.
Follow-up study time points were at 1, 3, 6, 9, and 12 months after stent insertion. Follow-up evaluation included uroflowmetry, RGU, plain radiography, and urinalysis. Success was defined as the absence of complications that required additional surgery and maintaining the urethral patency in RGU and uroflowmetry as well as patient's satisfaction. Because the currently available lengths of the Memokath stent range from 3 to 9 cm, we subdivided the patients into 3 groups according to the length of urethral stricture (shorter than 3 cm, between 3 and 9 cm, and longer than 9 cm) and compared the success rate between the groups. In addition, to analyze the outcomes according to the anatomical site of the urethral stricture, we subdivided the patients into 4 groups (bulbar, proximal penile, distal penile, and whole penile urethra) and compared the success rate between the groups. The success rate according to the length of the urethral stricture was compared by Mann-Whitney U test and the success rate according to the location of the urethral stricture was compared by Fisher exact test owing to the small number of patient cases included in this study. All p-values were two-sided, and p<0.05 was considered significant. Analyses were conducted by using IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA).
The mean age of the 13 patients was 47.7 years (range, 18 to 74 years). The mean stricture length was 5.54 cm (range, 1 to 12 cm). There were six patients with bulbar, four patients with proximal penile, one patient with distal penile, and two patients with whole penile urethral stricture, respectively. The mean operative time was 26.9 minutes (range, 18 to 48 minutes) (Table 1).
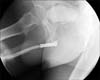
The overall success rate was 69% (9/13). All patients tolerated the stent with mild discomfort in 10 patients. Before stent placement, the mean peak flow rate was 5.23 mL/s (range, 0 to 12 mL/s). After stent insertion, the mean peak flow rate improved to 17.7 mL/s (range, 6 to 28 mL/s) at postoperative 1 day. Three patients had intermittent gross hematuria that was managed conservatively. Five patients had stress urinary incontinence that was controlled by Kegel exercises with or without an anticholinergic drug. Urethral hyperplasia was noted in three patients, and one of them required transurethral resection (Fig. 3). The stent was exchanged for one of a longer length (7 cm → 9 cm) in one patient, and another patient in whom two stents had been inserted required removal of the stent because of urethral obstruction between the two stents. One patient who underwent urethroplasty owing to penoscrotal-type hypospadias at 6 years of age had a skin perforation due to the stent and required removal of the stent (Fig. 4). Of 9 patients without major complications, uroflowmetry was performed at postoperative 6 months in 9 patients and at postoperative 12 months in 5 patients. The mean peak flow rates of each time point were 30 mL/s and 20.3 mL/s, respectively. RGU was performed at postoperative 3 months in 9 patients and at postoperative 9 months in 4 patients. All patients maintained urethral patency up to the last follow-up. Overall mean follow-up duration was 8.4 months and mean follow-up duration of patients with success was 7.2 months.
The Memokath stents come in different lengths from 3 cm to 9 cm in 1-cm increments. Therefore, patients were classified into groups according to stricture length of shorter than 3 cm, between 3 and 9 cm, and longer than 9 cm, and the success rates in these groups were 100% (5/5), 60% (3/5), and 33.3% (1/3), respectively. The shorter stricture group showed a higher success rate than did the longer stricture group, but the difference was not statistically significant (p=0.074) (Table 2). In the comparison of the success rate according to the stricture location, the success rate in bulbar and proximal penile urethral stricture patients was 83.3% (5/6) and 100% (4/4), respectively, whereas there were no successful cases in patients with distal and whole penile urethral strictures (0/1, 0/2, p=0.029) (Table 3).
Urethral stricture disease is one of the oldest and most problematic maladies known to urology and is common in current clinical practice. Urethral stricture disease and its treatment have been described from the time of the ancient Hindus, Egyptians, and Greeks [14,15]. Urethral stents were first introduced in 1980 by Fabian for treating infravesical obstruction owing to benign prostatic hyperplasia. The spring-shaped spiral coil inserted in the prostatic urethra keeps the enlarged prostate lobes compressing the urethra so far apart that spontaneous voiding is again possible [16]. Thermo-expandable urethral stents were introduced by Soni et al. [17] to treat patients with detrusor sphincter dyssynergia. Urethral stenting helped to achieve complete vesical emptying in 10 patients. With a follow-up of 3 to 7 months, all 10 patients remained asymptomatic, with residual urine of less than 50 mL.
Perry et al. [18] reported that most patients treated with the Memokath stent for bladder outlet obstruction of the prostatic urethra voided immediately after the procedure. Moreover, the reduction in International Prostate Symptom Score was immediate. Similarly, in the present study, there was appropriate stent function in all patients immediately after stent insertion, with an improvement in the peak flow rate and postvoid residual urine. Perry et al. [18] reported that the overall success rate was 63%. Jordan et al. [19] reported that 35 of 63 patients (56%) maintained urethral patency for 12 months after Memokath insertion. Our study showed that the overall success rate was 69% (9/13) for mean follow-up of over 8 months. This result adds information concerning the efficacy of the Memokath in that the urethral patency was maintained significantly longer than with catheter drainage alone.
The operative procedure of Memokath insertion is very simple and minimally invasive. In the present study, all patients underwent Memokath insertion after endoscopic visual urethrotomy; thus, every patient received spinal anesthesia. Memokath stent insertion can be performed as an outpatient procedure under local urethral anesthesia with instillation of intraurethral 2% lidocaine gel before the procedure [18]. Also, it does not require a postoperative recovery period. Jordan et al. [19] reported that the Memokath stent was inserted at the proper site with high accuracy under local anesthesia. Before the Memokath stent, the UroLume mesh type stent was used for the treatment of urethral stricture. However, removal of the UroLume stent is fraught with difficulty and can result in significant urethral injury and consequent stricture formation [20-22]. In contrast, the Memokath stent is densely coiled without mesh. It does not embed itself into the mucosa. Therefore, it is removable even after long-term indwelling. Mehta and Tophill [23] reported that the Memokath stent is readily removable with minimal trauma. In our study, the stents were removed in two patients owing to complications (fistula and urethral hyperplasia) and the removal procedure had no traumatic complications. The mean operative time of stent removal was 8.2 minutes.
One of the most common complications of urethral stents is urethral hyperplasia. Badlani et al. [24] reported that urethral hyperplasia was noted in 41.3% of patients who had received a UroLume stent. This is considered to be the result of urethral epithelium overgrowth through the interstices of the stent [13]. In the present study with the Memokath stent, urethral hyperplasia was noted at the ends of the stent in 3 patients (23%; Fig. 3). The low incidence of urethral hyperplasia with the Memokath stent was attributed to the tight coiling of the stent and the inert property of the nickel-titanium alloy from which it is made [13]. Eisenberg et al. [25] found that the most common surgical interventions required for failed urethral stents were transurethral resection of the hyperplasia (32%) and endoscopic litholapaxy for stent encrustation or stones (17%).
Up to now, urethrocutaneous fistula due to a stent had not yet been reported. In this study, a case of urethrocutaneous fistula due to the stent occurred in a 26-year-old patient who had undergone staged urethroplasty owing to penoscrotal-type hypospadias when he was 6 years old. The fistular site was primarily closed while the stent remained in the urethra at 1 month after stent insertion. However, the fistula occurred again, and the patient eventually underwent stent removal at postoperative 2 months. This case suggests that a prior history of urethroplasty may be a risk factor for urethrocutaneous fistula.
Jordan et al. [16] reported a rate of bacteriuria of 49.2%, and it could not be determined whether the bacteriuria represented only colonization of the stent or infection at some level proximal to the stent. In our study, a total of 3 cases (23%) of pyuria were observed. However, the patients did not have symptoms of urinary tract infection, such as dysuria or fever. They did not take prophylactic antibiotics and their stent function was maintained well during the study period. Perry et al. [18] reported an incidence of gross hematuria of only 3% in their cases. In contrast, our study showed gross hematuria in 23% (3/13) of the cases. We performed endoscopic internal urethrotomy by the cold knife procedure just before Memokath insertion in all patients. This procedure might have induced the higher rate of gross hematuria in our cases.
The failure rate in our study was 31% (4/13), and the stent was removed in two patients owing to complications (fistula and urethral hyperplasia). Of four patients with stent failure, the stent was changed to a longer one in one patient, and another patient underwent transurethral resection of hyperplastic urethral tissue. After those procedures, the stent function in these patients was well maintained in the study period. Among two patients who underwent stent removal, one patient with urethrocutaneous fistula was managed by intermittent urethral dilatation after fistula repair. His voiding function was maintained tolerably for 2 months of Memokath stent insertion and 3 months of intermittent urethral dilatation. However, the other patient who had a whole penile urethral stricture and was managed with the insertion of two stents suffered from urethral hyperplasia between the stents and all stents were removed. He still needs frequent urethral dilatation and catheterization. It is not certain if we can use the Memokath stent permanently or exactly how long the patients can keep the stent. Mehta and Tophill [23] reported that the Memokath stent has a "working life" of about 21 months and is not permanent because of complications. In this study, the mean time to occurrence of major complications was 7.15 months, and all patients except for the two stent removal cases have been tolerably maintaining the stents for more than 8 months. Low and McRae [22] described a cautionary experience of 26 stents inserted into 25 patients. In their study, although initial results appeared promising, most stents required removal owing to complications. Therefore, careful outpatient follow-up will be needed.
Basically, Memokath stent insertion is not contraindicated for penile urethral stricture. However, most studies about the Memokath stent for urethral stricture were limited to bulbar urethral strictures [13,19]. We experienced 7 cases of penile urethral strictures and showed a success rate of 42.9% (3/7). This is lower than our overall success rate and the success rate reported in other studies of bulbar urethral stricture. The urethral wall and cutaneous tissue are thinner in the penile urethral portion and stricture usually occurs with dense fibrosis in the penile urethra. It can cause more frequent stent migration, urethral hyperplasia, or even urethrocutaneous fistula. That may be one of the reasons for the high failure rate of the Memokath stent in penile urethra stricture. However, our study showed a success rate of 75% (3/4) within the limits of proximal penile urethral stricture. This result indicates that the Memokath stent can be applicable for proximal penile urethral stricture, although distal or whole penile urethral stricture has a higher risk of failure.
The limitations of this study are the relatively short follow-up duration, the small population, and the retrospective nature of the study. However, our study included the results of anterior urethral strictures as well as posterior urethral strictures. In addition, we reported our experience with various lengths of Memokath stents for not only short segment strictures but also long segment strictures, and this is the first study of Memokath stents for the management of urethral stricture in Korea. To determine the precise indications for the Memokath stent and to induce better efficacy, long-term follow-up and larger population-based randomized prospective study will be needed.
Thermo-expandable stents (Memokath) can be an alternative temporary management for recurrent urethral stricture patients who are unfit for or refuse urethroplasty. However, patients with distal penile urethral stricture or whole urethral stricture can have poor results. Although long-term follow-up with a larger patient population-based study will be needed, our study demonstrated that proximal urethral stricture or short segment stricture can be good indications for Memokath stent insertion.
Figures and Tables
 | FIG. 1(A) Single-ended Memokath (Pnn Medical, Kvistgaard, Denmark) stent. (B) Double-ended Memokath stent. |
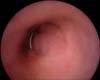 | FIG. 3Cystourethroscopic finding of urethral hyperplasia at distal penile urethra at postoperative 8 months. |
References
1. Santucci RA, Joyce GF, Wise M. Male urethral stricture disease. J Urol. 2007; 177:1667–1674.
2. Choi SH, Lee YS, Choi NG, Kim HJ. Initial experience with endoscopic holmium: YAG laser urethrotomy for incomplete urethral stricture. Korean J Urol. 2009; 50:246–250.
3. Lumen N, Hoebeke P, Willemsen P, De Troyer B, Pieters R, Oosterlinck W. Etiology of urethral stricture disease in the 21st century. J Urol. 2009; 182:983–987.
4. Bhargava S, Chapple CR. Buccal mucosal urethroplasty: is it the new gold standard? BJU Int. 2004; 93:1191–1193.
5. Bhargava S, Chapple CR, Bullock AJ, Layton C, MacNeil S. Tissue-engineered buccal mucosa for substitution urethroplasty. BJU Int. 2004; 93:807–811.
6. Pansadoro V, Emiliozzi P. Internal urethrotomy in the management of anterior urethral strictures: long-term followup. J Urol. 1996; 156:73–75.
7. Steenkamp JW, Heyns CF, de Kock ML. Internal urethrotomy versus dilation as treatment for male urethral strictures: a prospective, randomized comparison. J Urol. 1997; 157:98–101.
8. Greenwell TJ, Castle C, Andrich DE, MacDonald JT, Nicol DL, Mundy AR. Repeat urethrotomy and dilation for the treatment of urethral stricture are neither clinically effective nor cost-effective. J Urol. 2004; 172:275–277.
9. Heyns CF, Steenkamp JW, De Kock ML, Whitaker P. Treatment of male urethral strictures: is repeated dilation or internal urethrotomy useful? J Urol. 1998; 160:356–358.
10. Bullock TL, Brandes SB. Adult anterior urethral strictures: a national practice patterns survey of board certified urologists in the United States. J Urol. 2007; 177:685–690.
11. Andrich DE, Mundy AR. Urethral strictures and their surgical treatment. BJU Int. 2000; 86:571–580.
12. Anger JT, Buckley JC, Santucci RA, Elliott SP, Saigal CS. Urologic Diseases in America Project. Trends in stricture management among male Medicare beneficiaries: underuse of urethroplasty? Urology. 2011; 77:481–485.
13. Abdallah MM, Selim M, Abdelbakey T. Thermo-expandable metallic urethral stents for managing recurrent bulbar urethral strictures: to use or not? Arab J Urol. 2013; 11:85–90.
14. Chiou RK, Matamoros A, Anderson JC, Taylor RJ. Changing concepts of urethral stricture management. I: Assessment of urethral stricture disease. Nebr Med J. 1996; 81:282–286.
15. Webster GD, Koefoot RB, Sihelnik SA. Urethroplasty management in 100 cases of urethral stricture: a rationale for procedure selection. J Urol. 1985; 134:892–898.
16. Fabian KM. The intra-prostatic "partial catheter" (urological spiral) (author's transl). Urologe A. 1980; 19:236–238.
17. Soni BM, Vaidyanatham S, Krishnan KR. Use of Memokath, a second generation urethral stent for relief of urinary retention in male spinal cord injured patients. Paraplegia. 1994; 32:480–488.
18. Perry MJ, Roodhouse AJ, Gidlow AB, Spicer TG, Ellis BW. Thermo-expandable intraprostatic stents in bladder outlet obstruction: an 8-year study. BJU Int. 2002; 90:216–223.
19. Jordan GH, Wessells H, Secrest C, Squadrito JF Jr, McAninch JW, Levine L, et al. Effect of a temporary thermo-expandable stent on urethral patency after dilation or internal urethrotomy for recurrent bulbar urethral stricture: results from a 1-year randomized trial. J Urol. 2013; 190:130–136.
20. McFarlane IP, Foley SJ, Shah PJ. Long-term outcome of permanent urethral stents in the treatment of detrusor-sphincter dyssynergia. Br J Urol. 1996; 78:729–732.
21. Hamid R, Arya M, Patel HR, Shah PJ. The mesh wallstent in the treatment of detrusor external sphincter dyssynergia in men with spinal cord injury: a 12-year follow-up. BJU Int. 2003; 91:51–53.
22. Low AI, McRae PJ. Use of the Memokath for detrusor-sphincter dyssynergia after spinal cord injury: a cautionary tale. Spinal Cord. 1998; 36:39–44.
23. Mehta SS, Tophill PR. Memokath stents for the treatment of detrusor sphincter dyssynergia (DSD) in men with spinal cord injury: the Princess Royal Spinal Injuries Unit 10-year experience. Spinal Cord. 2006; 44:1–6.
24. Badlani GH, Press SM, Defalco A, Oesterling JE, Smith AD. Urolume endourethral prosthesis for the treatment of urethral stricture disease: long-term results of the North American Multicenter UroLume Trial. Urology. 1995; 45:846–856.
25. Eisenberg ML, Elliott SP, McAninch JW. Preservation of lower urinary tract function in posterior urethral stenosis: selection of appropriate patients for urethral stents. J Urol. 2007; 178:2456–2460.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download