Abstract
Purpose
To evaluate the safety and feasibility of a simplified zero ischemia technique using kidney donor computed tomographic (CT) angiography and conventional laparoscopic bulldog clamps.
Materials and Methods
We conducted a review of seven robot-assisted partial nephrectomies (RAPNs) performed by a single surgeon from January 2012 to May 2012. Using a simplified protocol of 3-dimentional reconstruction, tertiary arterial branches supplying the tumor were selectively clamped prior to resection. We used conventional laparoscopic bulldog clamps instead of microsurgical vessel clamps. The patients' demographic information, perioperative outcomes, pathologic outcomes and pre- and postoperative renal functions up to 3 months follow-up were analyzed.
Results
RAPN were successfully performed for seven complex renal hilar tumors. There were no significant differences in the total operation time, estimated blood loss or postoperative outcomes compared with published literature on standard RAPN. Negative surgical margins were reported in all cases.
Conclusions
We presented a simplified-zero ischemia technique using kidney Donor CT angiography and conventional laparoscopic bulldog clamps. We have also demonstrated its safety and feasibility in patients with complex renal hilar tumors. This modified technique can be easily adopted by most surgeons who are currently performing RAPN.
Minimally invasive partial nephrectomy for small renal masses was reported to have excellent functional and oncologic outcomes, with 5 to 10 years cancer-specific survival rates of 95% to 100% [1-4]. Among the various factors that are important for preserving renal function after partial nephrectomy, the most important factors are to maximize the amount of functional renal parenchyma [5], and to reduce warm ischemia time. In October 2010, the zero ischemia technique was introduced to eliminate global renal ischemia during RAPN [6]. According to the protocol, dedicated renal protocol computed tomographic (CT) scan with 0.5 mm slice thickness was essential to delineate tumor location, intrarenal extension, and proximity to collecting system. In addition, reconstruction of renal tumors were performed manually using mainly arterial phase images with the corresponding venous phases, and required the joint interpretation by an experienced urologist and a radiology technician. Furthermore, placement of a disposable neurovascular aneurysm microsurgical bulldog clamp (occlusion power, 0.39 N) on the targeted arterial branch was needed to confirm tumor devascularization [7].
Zero ischemia RAPN was a novel technique developed to bring about a paradigm shift from conventional partial nephrectomy. However, 2 years had lapsed since zero ischemia RAPN was first reported and there was a paucity of literature reporting on this technique. This may be attributed to the technically challenging nature of the procedure and the need for specialized instruments and equipment, making this approach less readily adopted by other centers.
In our present study, kidney donor CT angiography which is widely available in many centers is used to distinguish the tumor with its arterial anatomy from the surrounding normal parenchyma. Conventional laparoscopic bulldog clamps were applied to tertiary arteries supplying the tumour instead of disposable microsurgical clamps. The purpose of this study was to evaluate the safety and feasibility of a simplified zero ischemia technique using kidney donor CT angiography and conventional laparoscopic bulldog clamps in patients with renal hilar tumors.
A total of seven consecutive patients with enhancing renal masses with preoperatively reconstructed 3-dimentional (3D) CT images underwent robot-assisted partial nephrectomies (RAPNs) by a single experienced surgeon in a tertiary institution between January 2012 and May 2012. Institutional Review Board approval was obtained.
Preoperative imaging was done using a 64 slice multidetector-row CT scanner (GE Medical Systems, Milwaukee, WI, USA). Triphasic CT scanning consisting of non-contrasted, arterial, and cortico-medullary phases, with 1 mm slice thickness was performed. Using the standard commercial workstation of the CT scanner (AW volume-share 2, GE Medical Systems), the radiologist reconstructed 3D images to identify the renal arterial anatomy and the relationship between the renal artery and the tumor within the renal parenchyma. We reconstructed three components using a surface rendering technique and then fused them into a 3D volume data set: the renal artery, renal parenchyma, and tumor. The renal artery and tumor were reconstructed from images during the arterial phase. The total time required for reconstruction was one hour per case. The fused 3D volume-rendered data-set was viewable at all angles during preoperative planning (Figs. 1B, 2B).
The ports were placed transperitoneally. After the colon was taken down, the perirenal fat was meticulously dissected to reveal the renal hilar vessels in the retroperitoneal space. Except for clamping the higher order renal artery, the operative procedures of zero ischemia RAPN procedures were basically identical to those of conventional RAPN [8]. After hilar dissection, the artery feeding the tumor that was revealed by the 3D reconstruction images was carefully dissected and isolated using small silicone vessel loops (Aspen Surgical Products, Caledonia, MI, USA) (Fig. 2A). With traction of small silicone vessel loop on the tumor feeding artery, the absence of blood flow in the tumour and the tumour depth were confirmed with intraoperative Doppler ultrasonography. Resection margins were then delineated with intraoperative ultrasonography, especially for endophytic masses. The tertiary renal artery was selectively clamped with small conventional laparoscopic bulldog clamps (Atraumatic endo vessel clips, Braun Aesculap, Germany) (Fig. 2B). In all seven cases, clamping of the main renal artery was not performed. Radial incision of the parenchyma was not needed to locate the tertiary branches, and there was little venous backflow during tumor excision. In cases where there were bleeding from cut vessels on dissection of the renal parenchyma, compression of the vessel with the suction tip was performed by the assistant, so that the tumour margins remained clearly visible. The margins of resection were sent for frozen section pathologic analysis. The exposed collecting system and vessels were repaired with running 3-0 vicryl sutures. Second layer renorrhaphy was performed using the sliding Hemolok technique described by Benway et al. [9]. Surgical bolster was not interposed, but the suture line was covered with Surgicel (Ethicon, Somerville, NJ, USA) and Floseal (Baxter Inc., Deerfield, IL, USA). The laparoscopic bulldog clamps were removed after the first layer of renorrhaphy was completed.
Seven cases of RAPN were performed by a single experienced surgeon who had previous experience in more than 150 RAPN cases. Patient demographics, tumor characteristics and perioperative data are described in Table 1.
There was a high proportion high PADUA (preoperative aspects and dimensions used for an anatomical) [10] scored (>10) cancer (57.1%) and the mean radius, exophytic/endophytic, nearness to collecting system or sinus, anterior/posterior and location relative to polar lines (R.E.N.A.L.) nephrometry [11] and PADUA scores were relatively high. Four tumors were in the left kidney, and two were posterior hilar tumours. One patient had previous abdominal surgery.
Table 2 illustrates the pathological outcomes and complications. 6 tumors were clear cell renal cell carcinoma (RCC) and one unclassified. The unclassified RCC was reported as likely clear cell papillary type RCC, but was not perfectly compatible with the criteria by using various immunohistochemical stains. Surgical margins were negative in all cases. 4 renal tumors were of high Fuhrman grades and 1 tumor had invaded the perirenal fat. Repair of the pelvocalyceal system was performed in 6 cases and a bolster was not used in all seven cases. No patients had intraoperative or postoperative blood transfusions or severe complications. However, there were minor complications including one drain site wound infection, one urine leak and one postoperative fever that spontaneously subsided (Clavien classification I).
Data on renal function at 3 months postoperative were analysed in Table 3. Postoperative renal functions of each patient were similar to the preoperative renal functions. The mean estimated glomerular filtration rate (eGFR) percent change at 3 months postoperative was 5.8% (range, -17.1% to 15.27%) which was not statistically significant (p=0.393). As reported in Table 3, comparing to preoperative eGFR, the eGFR changes during each follow-up were not significantly different. The renal function recovery curve was shown in Fig. 3. Compared with other contemporary series of RAPN, our series seemed to have shorter total operative time and better eGFR, but higher estimated blood loss and hospital stay (Table 1).
Nephron sparing surgery has been accepted as the gold standard treatment for clinical stage T1 renal tumors [12]. Several studies reported that local recurrence and distant metastasis were not related to the width of resection margin [13,14]. Currently, minimally invasive techniques have been developed to reduce warm ischemic time and to preserve functional renal volume in partial nephrectomy [15]. These techniques include nonclamping method for exophytic small masses, early unclamping after excision of mass and suture techniques with nonhilar clamping [6,16]. Two years ago, Gill et al. [6] presented a technically challenging zero ischemia technique in an attempt to maximize renal function postoperatively. Zero ischemia RAPN is based on anatomical vascular microdissection of tertiary and higher order tumor feeding arterial branches. By scrutinizing 3D reconstructed images of the renal tumor, a neurosurgical aneurysm microbulldog (Bear disposable vascular clamp) was placed on the tumor specific arterial branch to selectively devascularize the tumor. However, acquiring the 3D reconstruction images was labor-intensive and time-consuming and had to be manually performed by an experienced urologist and a radiology technician and typically taking three hours. In addition, the application of the disposable microsurgical clamps was difficult and required technical expertise in laparoscopy. This was because these small clamps must be applied quickly when bleeding occurred. In addition, these clamps were easily mishandled and can be easily lost in the abdominal space.
Our vascular microdissection techniques were similar to the zero ischemia technique reported by Gill et al. [6]. However, we modified our techniques and protocol to overcome two challenges of Gill et al.'s techniques. Firstly, we utilized a conventional kidney Donor CT angiography which is readily available in many centers to provide 3D images and video clips using 1 mm slices (Fig. 1). The reconstruction took an hour and was performed only by a radiology technician, decreasing the radiologist's and urologist's workloads. Secondly, we used standard conventional laparoscopic bulldog clamps which were applied by the patient-side assistant for the super-selective clamping of tertiary arteries. For the safety reason, these were also tagged with threads so that they will not be lost in the abdomen in the event of accidental slippage. These changes made the technique more readily accessible for most centers already performing robotic partial nephrectomies. To prove the feasibility and safety of our technique, this study analysed seven cases of simplified zero ischemia RAPN in a high volume tertiary institution.
The main goal of zero ischemia technique is to avoid global renal ischemia and reperfusion injury, which causes renal function deterioration. Rogers et al. [17] reported their RAPN series for hilar tumors in 11 patients with a mean warm ischemia time of 28.9 minutes and mean postoperative eGFR change of -8 mL/min/1.73 m2. Abreu et al. [16] reported their robotic partial nephrectomy series of 7 patients with hilar tumours, with mean R.E.N.A.L. score of 9.4. The postoperative eGFR change was 1.3 mL/min/1.73 m2, mean hospital stay was 4.1 days and the total operation time was 237 minutes. In our present study, we reported better total operation time and absolute renal function change, but longer hospital stay and lower nephrometry scores.
In comparing length of hospitalization, the practical effect of Korean national health insurance system has to be considered. As most of the patients' medical expenses during hospitalization were reimbursed, the majority of patients in Korea do not return home until they could resume normal activities.
Despite the small numbers, this study showed that our simplified zero ischemia technique offered good perioperative outcomes. We postulate that ischemic injury to the peritumoral normal parenchyma was truly minimized. In addition, there were no severe complications and positive surgical margins in our series.
We showed that zero ischemia RAPN can be safely performed in patients with hilar tumors, even in highly complex (PADUA scores≥9) tumours. Patients with compromised renal function and young patients who have long life expectancy are good candidates for zero ischemia RAPN.
Interventional radiology expertise may not be available in many institutions. Selective clamping of the tumor feeding artery could prevent bleeding from the resected margins and reduces global reperfusion injuries. However the technical skills involved in skeletonizing the renal artery branches and selectively occluding them are challenging. The purpose of this paper is to provide a simplified and more easily adoptable version of the zero ischemia technique. Even though the present study comprises of a small number, we showed that our simplified technique is safe and feasible. The time and expertise needed for the acquisition of the 3D reconstructed images is reduced and can be reproduced in many centers as the donor CT angiography, which we used in our protocol, is widely available. Conventional laparoscopic bulldog clamps can be easily and safely applied as well. Furthermore, we showed that our simplified zero ischemia technique could be successfully performed in highly complex hilar tumors (PADUA score 11) with well-preserved postoperative renal functions and minimal complications.
Our zero ischemia technique can be used by most surgeons who are already performing RAPN. Longer term and larger randomized studies will be needed before consensus for the indications and benefits of zero ischemia partial nephrectomy can be established.
This study demonstrated the safety and feasibility of a simplified zero ischemia RAPN technique. In cases of complex renal hilar tumors, our initial experience revealed no intraoperative complications, as well as optimally preserving post-operative renal functions compared to contemporary series. Zero ischemia is a valuable technique that can shift the current paradigm of partial nephrectomy. Starting with the present study, this technically challenging procedure requires more simplification and availability.
Figures and Tables
FIG. 1
Three-Dimensional (3D) reconstruction image from computed tomographic (CT) angiography. (A) Kidney Donor CT angiography for a renal mass that has a PADUA (preoperative aspects and dimensions used for an anatomical) score of 11. (B) 3D reconstitution image (1 mm slice source) showed the tumor-specific tertiary arteries (yellow arrow) with multi directional image (kidney-blue, renal mass-yellow, vessel-red). There is another small tumor-specific first order artery that appeared to supply the tumor, but intraoperative Doppler sonography confirmed that it does not supply the tumour; this artery was not clamped during the operation (blue arrow).
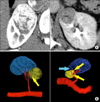
FIG. 2
Intraoperative vascular dissection, selection of tumor-specific arterial branches using color Doppler ultrasonography. (A) Tumor feeding artery (yellow arrow) arising from the tertiary order artery (blue arrow). Red arrow indicates the secondary order artery arising from the main renal artery. (B) Intraoperative color-Doppler ultrasound showing the peritumoral Doppler flow (left figure), which decreased (right figure) when the tumor-supplying artery was clamped with laparoscopic bulldog clamps.
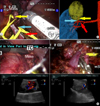
FIG. 3
Postoperative renal function at 3-month follow-up in patients who underwent simplified zero ischemia robotic partial nephrectomy. eGFR, estimated glomerular filtration rate; Preop, preoperation; Postop, postoperation; POD, postoperative day.

TABLE 1
Patient demographics, tumor characteristics and perioperative data

Values are presented as mean (range) or number (%).
ASA, American Society of Anesthesiologists; R.E.N.A.L., radius, exophytic/endophytic, nearness to collecting system or sinus, anterior/posterior and location relative to polar lines; PADUA, preoperative aspects and dimensions used for an anatomical.
ACKNOWLEDGMENTS
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2012 (6-2012-0181).
References
1. Antonelli A, Zani D, Cozzoli A, Nicolai M, Zanotelli T, Perucchini L, et al. Nephron-sparing surgery versus radical nephrectomy in the treatment of renal cell carcinoma up to 7 cm. Urologia. 2007. 74:173–179.
2. Joniau S, Vander Eeckt K, Srirangam SJ, Van Poppel H. Outcome of nephron-sparing surgery for T1b renal cell carcinoma. BJU Int. 2009. 103:1344–1348.
3. Peycelon M, Hupertan V, Comperat E, Renard-Penna R, Vaessen C, Conort P, et al. Long-term outcomes after nephron sparing surgery for renal cell carcinoma larger than 4 cm. J Urol. 2009. 181:35–41.
4. Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001. 166:6–18.
5. Simmons MN, Hillyer SP, Lee BH, Fergany AF, Kaouk J, Campbell SC. Functional recovery after partial nephrectomy: effects of volume loss and ischemic injury. J Urol. 2012. 187:1667–1673.
6. Gill IS, Eisenberg MS, Aron M, Berger A, Ukimura O, Patil MB, et al. "Zero ischemia" partial nephrectomy: novel laparoscopic and robotic technique. Eur Urol. 2011. 59:128–134.
7. Ng CK, Gill IS, Patil MB, Hung AJ, Berger AK, de Castro Abreu AL, et al. Anatomic renal artery branch microdissection to facilitate zero-ischemia partial nephrectomy. Eur Urol. 2012. 61:67–74.
8. Jeong W, Park SY, Lorenzo EI, Oh CK, Han WK, Rha KH. Laparoscopic partial nephrectomy versus robot-assisted laparoscopic partial nephrectomy. J Endourol. 2009. 23:1457–1460.
9. Benway BM, Wang AJ, Cabello JM, Bhayani SB. Robotic partial nephrectomy with sliding-clip renorrhaphy: technique and outcomes. Eur Urol. 2009. 55:592–599.
10. Ficarra V, Novara G, Secco S, Macchi V, Porzionato A, De Caro R, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol. 2009. 56:786–793.
11. Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009. 182:844–853.
12. Ljungberg B, Hanbury DC, Kuczyk MA, Merseburger AS, Mulders PF, Patard JJ, et al. Renal cell carcinoma guideline. Eur Urol. 2007. 51:1502–1510.
13. Sutherland SE, Resnick MI, Maclennan GT, Goldman HB. Does the size of the surgical margin in partial nephrectomy for renal cell cancer really matter? J Urol. 2002. 167:61–64.
14. Puppo P, Introini C, Calvi P, Naselli A. Long term results of excision of small renal cancer surrounded by a minimal layer of grossly normal parenchyma: review of 94 cases. Eur Urol. 2004. 46:477–481.
15. Funahashi Y, Hattori R, Yamamoto T, Kamihira O, Kato K, Gotoh M. Ischemic renal damage after nephron-sparing surgery in patients with normal contralateral kidney. Eur Urol. 2009. 55:209–215.
16. Abreu AL, Gill IS, Desai MM. Zero-ischaemia robotic partial nephrectomy (RPN) for hilar tumours. BJU Int. 2011. 108(6 Pt 2):948–954.
17. Rogers CG, Metwalli A, Blatt AM, Bratslavsky G, Menon M, Linehan WM, et al. Robotic partial nephrectomy for renal hilar tumors: a multi-institutional analysis. J Urol. 2008. 180:2353–2356.
18. Simmons MN, Ching CB, Samplaski MK, Park CH, Gill IS. Kidney tumor location measurement using the C index method. J Urol. 2010. 183:1708–1713.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download