Abstract
The incidence of erectile dysfunction (ED) increases with age and cardiovascular disease risk factors, such as hypertension, hyperlipidemia, insulin resistance, obesity, and diabetes. These risk factors are thought to contribute to endothelial dysfunction and atherosclerosis, thus contributing to the pathophysiology of ED. The role of the endothelium in regulating erectile physiology is well established. However, the role of androgens in modulating endothelial function and endothelial repair mechanisms subsequent to vascular injury in erectile tissue remains a subject of intensive research. The clinical and preclinical evidence discussed in this review suggests that androgens regulate endothelial function and also play an important role in the development and maturation of endothelial progenitor cells (EPCs), which are thought to play a critical role in repair of endothelial injury in vascular beds. In this review, we discuss the data available on the effects of androgens on endothelial function and EPCs in the repair of vascular injury. Indeed, more research is needed to fully understand the molecular and cellular basis of androgen action in regulating the development, differentiation, maturation, migration, and homing of EPCs to the site of injury. A better understanding of these processes will be critical to the development of new therapeutic approaches to the treatment of vascular ED.
Erectile function is a complex neurovascular process that requires the integration of a host of signaling pathways, leading to increased blood inflow to the corpora cavernosa and reduced blood outflow permitting entrapment of blood under pressure and engorgement of the penis [1-6]. Upon sexual stimulation, the release of neurotransmitters from the cavernosal nerves brings about vascular and trabecular smooth muscle relaxation. This permits increased blood flow from the cavernosal arteries and activation of endothelial cells by producing nitric oxide (NO) in response to shear stress. The relaxation of the vascular smooth muscle of the cavernosal artery, helicine arterioles, and the trabeculae increases intracavernosal pressure as the result of the accumulation of blood under pressure [1-6]. Vasodilation of the arterioles that feed the lacunar spaces and relaxation of corporeal smooth muscle, which surrounds these spaces, contributes to increased intracavernosal pressure and engorgement of the corporal bodies as a result of filling with blood, which results in expansion of the corpora bodies against the tunica albuginea. The accumulation of blood under pressure compresses the subtunical venules and impedes blood outflow, resulting in the entrapment of blood under pressure and a concomitant increase in the intracavernosal pressure, veno-occlusion, and subsequent erection.
The veno-occlusion mechanism is regulated by local release of 1) adrenergic and cholinergic neurotransmitters; 2) nonadrenergic, noncholinergic neurotransmitters; and 3) vasoactive agents produced by the vascular endothelium [3-5]. The sympathetic nervous system releases norepinephrine, which acts through the α-adrenergic receptor pathway, thus modulating the contractility of vascular and trabecular smooth muscles, leading to detumescence. Nonadrenergic, noncholinergic neurotransmitters (e.g., neural nitric oxide, vasoactive intestinal polypeptide) released from the parasympathetic nerves facilitate vascular and trabecular smooth muscle relaxation. In addition, NO and vasoactive substances, such as prostacyclins and prostaglandins, released by the vascular endothelium also stimulate smooth muscle relaxation, producing increased intracavernosal pressure, veno-occlusion, and erection [1-5,7]. Alterations or interferences in the mechanisms regulating vascular and trabecular smooth muscle contractility contribute to veno-occlusive dysfunction and ultimately erectile dysfunction (ED) [1,4,5].
Androgens play an important role in erectile physiology. It is believed that androgens are critical not only for the growth and function of the trabecular smooth muscle, but also in maintenance of the function of cavernosal and dorsal nerves as well as the function of the vascular endothelium. In this review, we focus mainly on the role of androgens in modulating endothelial function and in the development, differentiation, maturation, migration, and homing of endothelial progenitor cells (EPCs) and their role in maintaining erectile physiology.
The vascular endothelium plays a critical role in the vasculature [2,7-11]. The endothelium serves as a barrier between circulating blood and blood-borne elements and the surrounding tissues [8,12]. The endothelium is critical for angiogenesis and vascular repair. Furthermore, the vascular endothelium produces a host of regulatory factors, including NO, prostaglandins, endothelins (E-1, E-2, E-3), prostacyclin (PGI2), and angiotensin II, among others [8,13,14]. These signaling molecules regulate smooth muscle contractility and subsequent vasoconstriction and vasodilation and ultimately regulate blood flow to the penis. In erectile tissue, the signaling molecules produced by the endothelium regulate the contractility of the trabeculae and are deemed critical in normal erectile physiology [14]. Thus, the endothelium regulates vascular tone and erectile physiology via autocrine, paracrine, and endocrine mechanisms [7,13,14].
In erectile tissue, the endothelium lining the vascular bed and the trabeculae in the penis plays a critical role in erectile function. The increased blood flow through the cavernosal artery increases shear stress, which causes the endothelium to synthesize and release NO. NO relaxes the vascular smooth muscle and increases blood flow to the corpora cavernosa. The endothelium lining the lacunar spaces responds to this increased blood flow with increased synthesis and release of NO, which facilitates the relaxation of the trabecular smooth muscle of the corpora cavernosa. These signaling mechanisms originating from the endothelium are considered critical for the regulation of penile physiology and erections.
The endothelium responds rapidly to signaling by a variety of stimuli and is instrumental in the recruitment of immune and blood cells to sites of endothelial injury [1,7,8,14-17]. The endothelium regulates vascular homeostasis by activating platelets and inflammatory and immunological responses as well as by controlling vascular permeability, smooth muscle cell proliferation, and angiogenesis [1,7,14,18-20]. Thus, the endothelium plays an important role in the repair of injury to the vascular bed by replacing injured endothelial cells with mature EPCs. Baumhakel [21] investigated the relationship between circulating EPCs and erectile function by using CD133+ as a marker to identify the EPCs. Patients with ED had significantly lower levels of circulating CD133+ cells than did patients with normal erectile function. Circulating CD133+ cells showed an inverse correlation with ED, and increasing the number of these circulating EPCs resulted in a decreased likelihood of ED [21].
Endothelial dysfunction is associated with reduced endothelial nitric oxide synthase (eNOS) expression; increased production of the asymmetric dimethylarginine (ADMA); increased production of reactive oxygen species (ROS); increased synthesis and release of ET-1, E-2, and E-3; increased production of inflammatory cytokines such as tumor necrosis factor-α (TNF-α); increased expression of markers of cell adhesion such as E-selectin, intracellular adhesion molecule (ICAM), and vascular cell adhesion molecule (VCAM); deregulation of fibrinolytic factors such as von Willebrand factor (vWF); inability to regenerate endothelium from EPCs; increased endothelial cell apoptosis; increased cellular and vascular permeability; and increased vascular tone [22].
A common link underlying the pathophysiology of cardiovascular disease (CVD) and ED is vascular insufficiency attributed to endothelial dysfunction. A host of risk factors, including insulin resistance, type 2 diabetes mellitus, dyslipidemia, hypertension, obesity, metabolic syndrome, oxidative stress, atherosclerosis, smoking, and hyperhomocysteinemia, are thought to contribute significantly to endothelial dysfunction [14,22-27].
EPCs play an important role in vascular repair and angiogenesis and replacement of damaged endothelial cells in blood vessels [13-15,21,28-34]. EPCs were first described as a population of circulating cells able to engraft in areas of physiologic or pathological neovascularization, angiogenesis, and endothelial cell repair by acquiring an endothelial phenotype, expressing vWF, and incorporating Dil-Ac-LDL [8,13,15,28,35-38]. It is believed that endothelial injury is likely to be repaired by mature circulating EPCs.
EPCs are characterized as a population of CD34+ hematopoietic progenitor cells that can differentiate in vitro into an endothelial lineage, expressing a number of cell surface markers similar to those expressed by angioblasts and hematopoietic cells, which suggests a common precursor [15,28,29]. "Putative endothelial progenitor cells" isolated from human peripheral blood express two antigens, CD43 and fetal liver kinase (Flk-1). Peichev et al. [39] identified a CD133+/vascular endothelial growth factor receptor 2+ (VEGFR2+) population that differentiates into endothelial cells in vitro and is distinguishable from mature CD34+/VEGFR+ endothelial cells found on the vessel wall; note that the VEGFR is also known as kinase-inert domain receptor (KDR) [28,31,37,40].
The pathway by which EPCs originate in the bone marrow and a host of biochemical activators, which are thought to play a critical role in the development and differentiation of the precursors of circulating EPCs, are depicted in Fig. 1A. Briefly, the bone marrow produces hemangioblasts, which are thought to be a common precursor of hematopoietic stem cells as well as bone-marrow-derived angioblasts. The angioblasts undergo further differentiation in the presence of the appropriate activators into EPCs [30,31]. Fig. 1B illustrates the differentiation of early EPCs to late EPCs and the expression of various specific markers that are detected on the surface of the mature EPCs during circulation in the bloodstream.
Note that EPCs derived from bone marrow exhibit a wide range of heterogeneity; thus, there is no clear consensus on which specific antigenic profile or surface markers best identify EPCs [41]. However, the three most commonly used antigenic markers for the detection of EPCs are as follows: 1) CD34, an adhesion molecule; 2) CD133, a surface antigen with unknown function that is closely related to immature EPCs; and 3) Flk-1/KDR, or VEGF-2, which indicates early endothelial differentiation [41]. Other markers include AC133, CXCR4, and CD105 (Endoglins). It is believed that mature EPCs can home in on sites of endothelial injury and are involved in the vascular repair processes. Circulating progenitor cells represent a more immature pool of circulating cells with characteristics similar to those exhibited by EPCs. Both progenitor cells and EPCs originate from hematopoietic stem cells of the bone marrow, which then migrate into the peripheral circulation, home in on sites of neovascularization, and differentiate into mature endothelial cells and play a critical role in endothelial repair processes.
Several clinical trials have reported exciting findings regarding the potential use of EPCs in the treatment of various vascular disorders attributed to endothelial dysfunction. In the Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction trial (TOPCARE-AMI trial), infusion of ex vivo expanded bone marrow EPCs into patients with a history of myocardial infarction resulted in enhanced myocardial viability and increased ventricular ejection fraction [42]. EPCs have also been used diagnostically as biomarkers of disease detection or progression. For instance, a large number of studies have shown significant changes in the number and function of EPCs in diseases such as diabetes, hypercholesteremia, pulmonary hypertension, rheumatoid arthritis, and chronic renal disease [14,15,21,22,32,33,43, 44]. Also, reduced EPC number and altered proliferation and migration profiles have been correlated with smoking, aging, and chronic inflammation. These findings further support a critical role of EPCs in vascular repair mechanisms and homeostasis.
In the vasculature, androgens have been shown to modulate endothelial function via genomic and nongenomic mechanisms. In addition, androgens are the precursor for estrogens, which also modulate endothelial function [7,45,46]. Several studies have demonstrated that mature endothelial cells express androgen receptors (ARs), which suggests that androgens elicit a direct genomic-mediated action through the activation of the classic AR [7,47]. Androgens (testosterone and 5α-DHT) also promote nongenomic action via interactions with putative membrane ARs (nontranscriptionally) [7,46]. The results of preclinical and clinical studies have suggested that the relationship of endothelial function in response to androgen is complex and encompasses structural as well as cell signaling pathways. Testosterone deficiency (TD) has been shown to contribute to endothelial damage and dysfunction, and androgen therapy enhances endothelial repair [20,48-52]. Androgens increase the synthesis and release of NO in the vascular endothelium [2,48,53-55]. A recent report has suggested that androgens stimulate EPC proliferation via an AR/VEGF-mediated mechanism [56]. In addition, real-time real time quantitative reverse transcription polymerase chain reaction analysis showed that 5α-DHT induced AR, cyclin A, cyclin D1, and VEGF gene expression in a dose- and time-dependent manner [56]. In in vitro studies using the AR antagonist Casodex and specific AR siRNA, a marked reduction in 5α-DHT-induced endothelial cell proliferation and targeted gene expression was shown, which suggests that these effects are AR-dependent. In addition, inhibition of VEGF receptor signaling or expression produced a dose-dependent blockade of 5α-DHT-induced endothelial cell proliferation and cyclin A gene cell expression. These findings strongly suggest that androgens stimulate endothelial cell proliferation via upregulation of VEGF-A, cyclin A, and cyclin D1. Androgens also promote angiogenesis via cellular mechanisms involving VEGF, a specific cytokine that plays an important role in mitosis in the vasculature. Specifically, binding of VEGF to its receptors on endothelial cells promotes and enhances vascular permeability and facilitates angiogenesis and revascularization [40]. More interestingly, Godoy et al. [57] linked androgens to the upregulation of VEGF and mitotic cyclins, which are known to increase EPC proliferation [57]. In clinical studies it was demonstrated that flow-mediated dilation (FMD) is reduced in patients with TD and is enhanced in response to testosterone therapy [23,58-60].
Lu et al. [50] demonstrated that androgen deprivation by castration or by inhibition of the enzyme 5α-reductase resulted in profound physical and structural changes in endothelial structure in rat aorta as detected by transmission scanning electron microscopy analyses. In aortic tissues from control animals, the endothelium appeared smooth and freely compliant. In contrast, the endothelium in the aorta of castrated animals or intact animals treated with the 5α reductase irreversible inhibitor finasteride were severely crimpled, coarse, and protuberant, and the connections between cells were disrupted and many red blood cells were noted to adhere to the endothelial surface [50]. These findings suggest that not only testosterone but also 5α-DHT is critical for endothelial function. More importantly, the aortic endothelium from castrated animals treated with testosterone undecanoate showed marked structural recovery, suggesting vascular repair [50]. On the basis of these observations, it was suggested that androgen deprivation, via castration or treatment with 5α-reductase inhibitor, produced injuries in aortic endothelial cells [7,50]. This is an important finding that links TD to endothelial dysfunction and androgen therapy to endothelial repair. Because endothelial dysfunction contributes to ED, the latter is considered an early warning sign that precedes clinically significant CVD by several years [7,50].
In addition, several clinical studies have supported a role for androgen modulation of endothelial function. In a cross-sectional study, Akishita et al. [23] demonstrated that total and free testosterone levels in men were positively correlated with %FMD, a surrogate marker of clinical atherosclerosis, which also reflects endothelial function [59,60]. Akishita et al. [23] further suggested that total and free testosterone were related to %FMD, independent of age, body mass index, hypertension, hyperlipidemia, diabetes mellitus, and current smoking. Acute [58] and chronic [61] testosterone therapy in men with TD enhanced %FMD without affecting the basal diameter of the brachial artery, which suggests an endothelium-dependent vasodilator action of testosterone. The relationship between %FMD and testosterone was not altered after adjustment for percentage nitroglycerine-mediated dilation (%NMD), which further supports the action of testosterone on endothelial function. In another study of 722 men aged 25 to 85 years, FMD and NMD measurements were carried out and correlated with total and free testosterone levels. It was shown that lower serum total and free testosterone levels are associated with impaired endothelial function [62]. These findings corroborate those reported by Akishita et al. [23] and support the notion that testosterone has a positive role on vascular health [63] via the modulation of endothelial function and endothelial cell repair after injury.
It has been suggested that androgens modulate endothelial cell function and differentiation and the proliferation of EPCs by genomic and nongenomic mechanisms (Fig. 2) [7,45-47]. Testosterone or its active metabolite 5α-DHT bind to the classic AR and elicit genomic responses resulting in increased VEGF and cyclin expression as well as increased eNOS expression and activity. Androgens may also interact with membrane ARs and activate a signaling cascade that results in increased VEGF and matrix metallopeptidase-9 (MMP-9) expression, together with increased activity of eNOS resulting in increased differentiation and proliferation of EPCs from bone marrow stroma precursor cells [64,65].
Foresta et al. [44] investigated the relationship between serum testosterone in patients with hypogonadotropic hypogonadism and circulating levels of both progenitor cells and EPCs. Hypogonadotropic hypogonadism patients exhibited low levels of testosterone and also reduced circulating levels of progenitor cells and EPCs. These observations suggested that testosterone may modulate the differentiation, proliferation, and maturation of these cells. A significant increase in the number of progenitor cells and EPCs was noted subsequent to testosterone treatment [44]. In a subsequent study, the authors demonstrated that the increase in the proliferation, migration, and colony formation activity of EPCs induced by androgens is an AR-dependent pathway [66].
In Klinefelter syndrome patients, the number of circulating EPCs is markedly reduced [67]. Because EPCs have never been studied in this syndrome, the authors evaluated the number of circulating EPCs in 68 adult men with this syndrome (47, XXY) and in 46 healthy men. Patients and controls were divided into two subgroups according to the absence or presence of cardiovascular risk factors [67]. Controls without cardiovascular risk factors had significantly higher levels of EPCs than did controls with cardiovascular risk factors. By contrast, Klinefelter syndrome patients without cardiovascular risk factors had EPC levels similar to those of men with Klinefelter syndrome with risk factors and significantly lower than those of controls without cardiovascular risk factors [67]. The number of EPCs in patients with TD was not different from the number in patients with normal testosterone levels. Twenty-two patients with TD were reevaluated after 6 months of testosterone therapy, but the authors did not observe any modification in the number of EPCs [67]. These findings are contrary to those reported previously in hypogonadal men [44], which suggests that TD associated with Klinefelter syndrome may be confounded by other factors owing to the genetic nature of this disorder.
It should be noted that studies of low serum testosterone and its relation to EPCs vary in quality, with inconsistent findings. Because EPCs express the AR, it seems reasonable to suggest that androgens may influence EPC proliferation and migration. Some studies have reported that men with TD exhibited reduced total basal testosterone, estradiol (E2), luteinizing hormone (LH), and follicular stimulating hormone (FSH) levels, and demonstrated increased numbers of circulating EPCs in response to testosterone therapy [44]. After treatment, no changes in FSH or LH levels were noted; however, both E2 and testosterone levels had increased to normal, physiological values. To ascertain the role of testosterone in modulating EPCs, immunocytochemistry on cultured EPCs showed a stronger signal for AR expression in the nucleus and cytoplasm, which suggests a possible role of androgens in EPC proliferation. The magnitude of the response found may be linked to the restoration of normal testosterone levels given the demonstrated wide expression of AR in EPCs or it may be a corroboration of testosterone conversion to E2 by the enzyme aromatase. However, because both testosterone and E2 levels increased after testosterone administration, the peripheral conversion of testosterone to E2 could not be excluded as a reason for the increased EPC levels. As a follow-up study, EPCs were treated with a synthetic, nonaromatizable androgen in vitro and demonstrated increased migration and proliferation by an AR-mediated mechanism [66]. These findings provide strong support for a role of androgens in stimulating EPC generation, differentiation, and migration from the bone marrow stromal cells. Studies by Fadini et al. [65] demonstrated that androgens exerted no direct effect on late EPCs in vitro or in vivo and noted that androgen stimulation was only observed in early EPCs; no effects on late EPCs were noted.
Endothelial dysfunction is a major contributing factor to CVD and represents a shift from a healthy endothelium to a damaged procoagulative, proinflammatory, and provasoconstrictive endothelium [35]. An intact functional endothelium is critical in maintaining the vascular functions of arterial relaxation and venous constriction [68,69]. Endothelial dysfunction contributes to vascular stiffness, increased vascular tone, production of inflammatory cytokines, increased permeability, decreased endothelial cell growth, and dysregulation of fibrinolytic factors [22]. In addition, endothelial dysfunction results in reduced expression and activity of eNOS and increased production of ADMA, a competitive inhibitor of eNOS. Interestingly, in patients with idiopathic hypotrophic hypogonadism, elevated plasma ADMA levels are associated with a reduction in NO production and parenteral testosterone administration reduces ADMA concentrations and increases NO production, which suggests that androgens restore endothelial function [70].
Furthermore, endothelial dysfunction is also associated with increased production of ROS; increased synthesis and release of ET-1, E-2, and E-3; increased production of inflammatory cytokines such as TNF-α; increased expression of markers of cell adhesion such as E-selectin, ICAM, and VCAM; deregulation of fibrinolytic factors such as vWF; inability to regenerate endothelium from EPCs; increased endothelial cell apoptosis; increased cellular/vascular permeability; and increased vascular tone, as noted previously [22]. These pathophysiological mechanisms, coupled with a reduction in EPCs, could underpin a common pathogenesis for CVD and ED and endothelial dysfunction [22,21].
The balance between endothelial repair mechanisms and the extent of injury due to vascular insults may determine the degree or severity of vascular dysfunction. It has been postulated that circulating EPCs are integral to the repair process of the injured vascular beds. The degree of oxidative stress, inhibition of eNOS activity, and increased levels of ADMA and hyperhomocysteinemia are among some of the factors that contribute to endothelial damage. These same factors may impair vascular repair by altering the development, differentiation, or mobilization of EPCs. Therapeutic approaches that modify the vascular risk factors, reduce endothelial injury, and facilitate endothelial repair pathways, such as statin therapy [33,71,72] or androgen replacement therapy, in men with TD have shown a marked increase in circulating EPCs [15,44,65]. These studies suggest that EPCs play a pivotal role in vascular homeostasis and that androgens may have a profound role in modulating EPC differentiation, proliferation, and maturation. Furthermore, these studies suggest that androgens play a role in maintaining endothelial cell function and repair mechanisms.
Aging and associated comorbidities contribute to endothelial dysfunction by reducing the ability to protect the endothelium from injury and insult produced by dyslipidemia, insulin resistance, type 2 diabetes, inflammation, metabolic syndrome, obesity, hypertension, and oxidative stress [73]. Because many of these comorbidities are also associated with reduced testosterone levels [73,74], it is possible that TD is a major risk factor for endothelial dysfunction in men [49]. Fig. 3 relates TD and several comorbidities as they relate to endothelial injury. As men age, serum testosterone concentrations decrease by about 1% per year [23]. However, in the current literature, it is not fully understood whether this decrease in testosterone levels is mainly due to normal aging or whether it is related to hypogonadism, a decrease in the functionality of the hypothalamic-pituitary-gonadal axis. Recent studies have suggested that aging per se is not the risk factor for TD and that other risk factors such as obesity and metabolic syndrome may be the real contributors to hypogonadism [75,76]. Testosterone has important metabolic actions in men, including body composition, insulin sensitivity, and lipid metabolism [49,52,69,77,78]. Low testosterone levels are also associated with ED [48,49,77]. This is important because ED is a vascular condition, and because ED and CVD are closely related, ED can be the first clinical manifestation of underlying CVD and can serve as an early warning sign for primary care physicians [23,49,79]. Thus, it is possible to infer that reduced levels of testosterone negatively impact vascular dynamics and the reactivity of blood vessels (Fig. 4) [9,49,77,78].
The actions of regenerating and repairing areas of vascular insult due to ischemia or endothelial injury do not depend solely on the cells residing in the area of insult but are influenced by bone marrow cells [27,33,72,80]. EPCs are premature, circulating, bone marrow-derived cells with putative trans-differentiation potential, as was shown in Figs. 1, 2 [80]. The risk factors thought to be involved are illustrated in Fig. 4 and may lead to a decreased number of circulating EPCs [33,80]. Studies have also shown that men with CVD risk factors are likely to have lower testosterone, which is independently associated with endothelial dysfunction [7]. Men with congestive heart disease exhibit a high prevalence of low testosterone levels, irrespective of age [49]. Araujo et al. [81] suggested that cardiovascular mortality via endothelial dysfunction is driven by the underlying health status and that a low testosterone level is a marker of poor general health; however, low testosterone and disease pathology exist as concomitant risk factors [81]. Inflammation, obesity, and insulin resistance are known to reduce testosterone levels, and testosterone is known to confer beneficial effects on those risk factors, thus ameliorating CVD [20,23,49,52,77,78].
Reduced levels of testosterone may exacerbate obesity, insulin resistance, dyslipidemia, and hypertension, among others [7,49,77-79]. In recent studies, testosterone treatment for ED ameliorated metabolic syndrome components (such as insulin resistance) and improved endothelial function [48]. Furthermore, the administration of testosterone undecanoate therapy to hypogonadal men with ED and venous leakage ameliorated erectile function by improving veno-occlusion [48,82]. Testosterone may have a beneficial effect on the systemic vasculature by ameliorating ED, thus reinforcing the idea that testosterone supplementation could prevent or delay the progression of disease [7].
The Massachusetts Male Aging Study [23,83] confirmed that ED is highly correlated with coronary artery disease, hypertension, heart disease, and diabetes. Men with low levels of testosterone and without CVD also have decreased blood flow and reduced nitrate-dependent vasodilation [84]. Zitzmann [51] found that the number of the trinucleotides comprised of the repeat of cytosine, adenine and guanine repeats in the AR, which reduces the sensitivity to testosterone, was associated with endothelium-dependent and endothelium-independent brachial artery vasodilation in men, which shows the effect of the AR action on vasoreactivity [49].
The common denominator among the underlying pathophysiology of ED and CVD is vascular insufficiency promoted by atherosclerosis [85,86] via endothelial dysfunction and oxidative stress from various metabolic conditions [24]. An increase in circulating EPCs is thought to be related to the bioavailability of NO, including increased activity of eNOS, anti-inflammatory and antioxidative enzymes, and the activation of MMP-9 [87]. Furthermore, because testosterone deprivation decreases the availability of NO [66], and because NO is a key marker of endothelial function, it can be inferred that testosterone has a direct effect on the endothelium or circulating EPCs via an increase in NO [66,88]. Burger and Touyz [35] showed that healthy endothelial cells possess certain cellular biomarkers, and dysfunctional endothelium exhibits impaired vasodilator synthesis and release, such as of NO and PGI2, and an increase in ROS [35]. Yu et al. [89] have also shown the benefits of testosterone in activated eNOS in vascular endothelial cells, thereby increasing the amount of NO in the endothelium.
Additionally, Hamed et al. [25] showed that men with type 2 diabetes showed a positive correlation between circulating NO and circulating EPCs. This is in direct affirmation of Burger and Touyz [35]'s study, which stated that a dysfunctional endothelium has low concentrations of NO. Hyperglycemia produces ROS, which then decrease NO availability [25]. Hyperglycemia can conjure ROS buildup via increased PKC/NADPH oxidase activity, which can lead to an uncoupling of eNOS and reduced NO availability, thus impairing the number of progenitor cells and their subsequent function [25]. It is known that deregulation of vascular tone, or endothelial dysfunction, is characterized by decreased availability of NO, which inadvertently induces the immune system to release white blood cells to the site of vascular deregulation [90]. This is of particular importance because of the role testosterone plays in NO production and maintenance. Shabsigh et al. [53] demonstrated in animal studies that the expression of various NOS isoforms in the corpora cavernosum is regulated by androgens. Also, NOS activity and protein expression are decreased in castrated animals, and testosterone administration restores and normalizes NOS protein expression and activity, allowing mobilization and homing of EPCs to areas of vasculogenesis and angiogenesis [53].
There remains an inextricable link between the role of testosterone and ED; however, this link provokes some controversy owing to the multifactorial nature of the pathophysiology of ED. Testosterone is critical to the health of the vascular beds [49,56], stimulating endothelial cell proliferation via an AR/VEGF-mediated mechanism, and promoting angiogenesis [56]. Furthermore, because testosterone deprivation is known to decrease the availability of NO [66], and because NO is a key marker of endothelial function, it can be inferred that testosterone may have a direct effect on endothelial function and development and the differentiation, maturation, migration, and homing of circulating EPCs.
Although the role of androgens in modulating endothelial function and in the development and differentiation of progenitor cells is not fully understood, several studies have shown that testosterone has a positive effect on the endothelium and improves the number of EPCs. More molecular and biochemical studies are warranted to delineate the exact mechanisms by which testosterone regulates endothelial function. The arena of EPCs and their role in vascular repair and treatment of disease is an emerging field, and the role of testosterone in this physiological process will require more detailed investigations. Cellular and molecular understanding of these processes will unlock new therapeutic approaches for the treatment of ED and CVD in men.
Figures and Tables
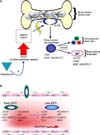 | FIG. 1(A) Potential pathway of bone marrow origin of endothelial progenitor cells and the various triggers and cell surface antigens of the putative endothelial cells. According to current knowledge in the literature, testosterone upregulates vascular endothelial growth factor (VEGF), endothelial nitric oxide synthase (eNOS), metallopeptidase-9 (MMP-9), and other modulators of endothelial progenitor cell (EPC) proliferation. The bone marrow hemangioblast is a common precursor of both hematopoietic stem cells and bone marrow-derived angioblasts. The bone marrow angioblasts further differentiate into endothelial progenitor cells. (B) Differentiation of early and late endothelial progenitor cells and appearance of various cell surface antigens. The exact cell antigens associated with early and late EPCs are not fully understood. In this diagram, we present a schematic of early and late EPCs on the basis of a review of the scientific literature. Both phenotypes exhibit kinase-insert domain receptor (KDR) antigen, whereas CD-133, von Willebrand factor (vWF), and endothelial nitric oxide synthase (eNOS) appear to be markers for a more mature progenitor cell type. c-Kit, proto-oncogene c-Kit; m-KitL, Murine Kit ligand. |
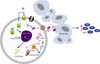 | FIG. 2A proposed role of androgens in regulating endothelial progenitor cell (EPC) proliferation from stromal cells. Testosterone (T) binds to androgen receptors (ARs) on the cell membrane or diffuses into the cell and binds to the intracellular classic AR. The AR ligand complex may exert genomic effects by upregulating vascular endothelium growth factor (VEGF) and mitotic cyclins or by increasing the expression of matrix metallopeptidase-9 (MMP-9) and nitric oxide (NO) in the cytoplasm. T may also have nongenomic effects by directly activating a cascade of signaling resulting in increased NO and VEGF. All of these molecules potentially increase the proliferation of EPCs from the bone marrow. eNOS, endothelial nitric oxide synthase; sGC, soluble guanylyl cyclase; GTP, guanosine triphosphate; GMP, guanosine monophosphate; PDE, phosphodiesterase. |
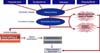 | FIG. 4Vascular endothelial injury and the pathophysiology of erectile dysfunction. In erectile dysfunction owing to arterial insufficiency, a lower oxygen tension is recorded in corporeal blood, which can lead to veno-occlusive dysfunction. This schematic diagram further suggests the role of the vascular endothelium in erectile physiology and pathophysiology. Adapted from Saenz de Tejada, et al. J Sex Med 2005;2:26-39 [6]. |
References
1. Andersson KE. Erectile physiological and pathophysiological pathways involved in erectile dysfunction. J Urol. 2003; 170(2 Pt 2):S6–S13.
2. Azadzoi KM, Kim N, Brown ML, Goldstein I, Cohen RA, Saenz de Tejada I. Endothelium-derived nitric oxide and cyclooxygenase products modulate corpus cavernosum smooth muscle tone. J Urol. 1992; 147:220–225.
3. Kim N, Azadzoi KM, Goldstein I, Saenz de Tejada I. A nitric oxide-like factor mediates nonadrenergic-noncholinergic neurogenic relaxation of penile corpus cavernosum smooth muscle. J Clin Invest. 1991; 88:112–118.
4. Saenz de Tejada I, Goldstein I, Krane RJ. Local control of penile erection. Nerves, smooth muscle, and endothelium. Urol Clin North Am. 1988; 15:9–15.
5. Saenz de Tejada I, Kim N, Lagan I, Krane RJ, Goldstein I. Regulation of adrenergic activity in penile corpus cavernosum. J Urol. 1989; 142:1117–1121.
6. Saenz de Tejada I, Angulo J, Cellek S, Gonzalez-Cadavid N, Heaton J, Pickard R, et al. Pathophysiology of erectile dysfunction. J Sex Med. 2005; 2:26–39.
7. Castela A, Vendeira P, Costa C. Testosterone, endothelial health, and erectile function. ISRN Endocrinol. 2011; 2011:839149.
8. Rosenzweig A. Endothelial progenitor cells. N Engl J Med. 2003; 348:581–582.
9. Traish AM, Abu-Zahra H, Guay AT. The brain, the penis and steroid hormones: clinical correlates with endothelial dysfunction. Curr Pharm Des. 2008; 14:3723–3736.
10. Fazio L, Brock G. Erectile dysfunction: management update. CMAJ. 2004; 170:1429–1437.
11. Vercellotti GM, Moldow CF, Jacob HS. Complement, oxidants, and endothelial injury: how a bedside observation opened a door to vascular biology. J Clin Invest. 2012; 122:3044–3045.
12. Foresta C, Di Mambro A, Caretta N, De Toni L, Zuccarello D, Ferlin A. Effect of vardenafil on endothelial progenitor cells in hypogonadotrophic hypogonadal patients: role of testosterone treatment. Clin Endocrinol (Oxf). 2009; 71:412–416.
13. Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003; 23:1185–1189.
14. Bivalacqua TJ, Usta MF, Champion HC, Kadowitz PJ, Hellstrom WJ. Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J Androl. 2003; 24:6 Suppl. S17–S37.
15. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003; 348:593–600.
16. Andersson KE, Wagner G. Physiology of penile erection. Physiol Rev. 1995; 75:191–236.
17. Traish AM, Goldstein I, Kim NN. Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction. Eur Urol. 2007; 52:54–70.
18. Bivalacqua TJ, Usta MF, Champion HC, Leungwattanakij S, Dabisch PA, McNamara DB, et al. Effect of combination endothelial nitric oxide synthase gene therapy and sildenafil on erectile function in diabetic rats. Int J Impot Res. 2004; 16:21–29.
19. Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005; 32:379–395.
20. Traish AM, Guay A, Feeley R, Saad F. The dark side of testosterone deficiency: I. Metabolic syndrome and erectile dysfunction. J Androl. 2009; 30:10–22.
21. Baumhakel M, Werner N, Bohm M, Nickenig G. Circulating endothelial progenitor cells correlate with erectile function in patients with coronary heart disease. Eur Heart J. 2006; 27:2184–2188.
22. Aversa A, Bruzziches R, Francomano D, Natali M, Gareri P, Spera G. Endothelial dysfunction and erectile dysfunction in the aging man. Int J Urol. 2010; 17:38–47.
23. Akishita M, Hashimoto M, Ohike Y, Ogawa S, Iijima K, Eto M, et al. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res. 2007; 30:1029–1034.
24. Guay AT. ED2: erectile dysfunction=endothelial dysfunction. Endocrinol Metab Clin North Am. 2007; 36:453–463.
25. Hamed S, Brenner B, Roguin A. Nitric oxide: a key factor behind the dysfunctionality of endothelial progenitor cells in diabetes mellitus type-2. Cardiovasc Res. 2011; 91:9–15.
26. Tomada N, Tomada I, Botelho F, Pacheco-Figueiredo L, Lopes T, Negrao R, et al. Endothelial function in patients with metabolic syndrome and erectile dysfunction: a question of angiopoietin imbalance? Andrology. 2013; 1:541–548.
27. Pelliccione F, D'Angeli A, Filipponi S, Falone S, Necozione S, Barbonetti A, et al. Serum from patients with erectile dysfunction inhibits circulating angiogenic cells from healthy men: relationship with cardiovascular risk, endothelial damage and circulating angiogenic modulators. Int J Androl. 2012; 35:645–652.
28. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999; 85:221–228.
29. Basile DP, Yoder MC. Circulating and tissue resident endothelial progenitor cells. J Cell Physiol. 2014; 229:10–16.
30. Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med (Berl). 2004; 82:671–677.
31. Garmy-Susini B, Varner JA. Circulating endothelial progenitor cells. Br J Cancer. 2005; 93:855–858.
32. Garolla A, D'Inca R, Checchin D, Biagioli A, De Toni L, Nicoletti V, et al. Reduced endothelial progenitor cell number and function in inflammatory bowel disease: a possible link to the pathogenesis. Am J Gastroenterol. 2009; 104:2500–2507.
33. Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001; 103:2885–2890.
34. Koutroumpi M, Dimopoulos S, Psarra K, Kyprianou T, Nanas S. Circulating endothelial and progenitor cells: Evidence from acute and long-term exercise effects. World J Cardiol. 2012; 4:312–326.
35. Burger D, Touyz RM. Cellular biomarkers of endothelial health: microparticles, endothelial progenitor cells, and circulating endothelial cells. J Am Soc Hypertens. 2012; 6:85–99.
36. Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004; 24:288–293.
37. La Vignera S, Condorelli R, Vicari E, D'Agata R, Calogero A. Original immunophenotype of blood endothelial progenitor cells and microparticles in patients with isolated arterial erectile dysfunction and late onset hypogonadism: effects of androgen replacement therapy. Aging Male. 2011; 14:183–189.
38. Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998; 92:362–367.
39. Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000; 95:952–958.
40. Gou X, He WY, Xiao MZ, Qiu M, Wang M, Deng YZ, et al. Transplantation of endothelial progenitor cells transfected with VEGF165 to restore erectile function in diabetic rats. Asian J Androl. 2011; 13:332–338.
41. Esposito K, Ciotola M, Maiorino MI, Giugliano F, Autorino R, De Sio M, et al. Circulating CD34+ KDR+ endothelial progenitor cells correlate with erectile function and endothelial function in overweight men. J Sex Med. 2009; 6:107–114.
42. Schächinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004; 44:1690–1699.
43. Barkin J. Erectile dysfunction and hypogonadism (low testosterone). Can J Urol. 2011; 18:Suppl. 2–7.
44. Foresta C, Caretta N, Lana A, De Toni L, Biagioli A, Ferlin A, et al. Reduced number of circulating endothelial progenitor cells in hypogonadal men. J Clin Endocrinol Metab. 2006; 91:4599–4602.
45. Herrmann JL, Abarbanell AM, Weil BR, Manukyan MC, Poynter JA, Wang Y, et al. Gender dimorphisms in progenitor and stem cell function in cardiovascular disease. J Cardiovasc Transl Res. 2010; 3:103–113.
46. Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002; 16:2181–2187.
47. Schultheiss D, Badalyan R, Pilatz A, Gabouev AI, Schlote N, Wefer J, et al. Androgen and estrogen receptors in the human corpus cavernosum penis: immunohistochemical and cell culture results. World J Urol. 2003; 21:320–324.
48. Saad F, Gooren L, Haider A, Yassin A. Effects of testosterone gel followed by parenteral testosterone undecanoate on sexual dysfunction and on features of the metabolic syndrome. Andrologia. 2008; 40:44–48.
49. Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013; 217:R47–R71.
50. Lu YL, Kuang L, Zhu H, Wu H, Wang XF, Pang YP, et al. Changes in aortic endothelium ultrastructure in male rats following castration, replacement with testosterone and administration of 5alpha-reductase inhibitor. Asian J Androl. 2007; 9:843–847.
51. Zitzmann M, Brune M, Kornmann B, Gromoll J, von Eckardstein S, von Eckardstein A, et al. The CAG repeat polymorphism in the AR gene affects high density lipoprotein cholesterol and arterial vasoreactivity. J Clin Endocrinol Metab. 2001; 86:4867–4873.
52. Zitzmann M. Testosterone deficiency and treatment in older men: definition, treatment, pitfalls. Asian J Androl. 2010; 12:623–625.
53. Shabsigh R, Rajfer J, Aversa A, Traish AM, Yassin A, Kalinchenko SY, et al. The evolving role of testosterone in the treatment of erectile dysfunction. Int J Clin Pract. 2006; 60:1087–1092.
54. Baba K, Yajima M, Carrier S, Morgan DM, Nunes L, Lue TF, et al. Delayed testosterone replacement restores nitric oxide synthase-containing nerve fibres and the erectile response in rat penis. BJU Int. 2000; 85:953–958.
55. Chamness SL, Ricker DD, Crone JK, Dembeck CL, Maguire MP, Burnett AL, et al. The effect of androgen on nitric oxide synthase in the male reproductive tract of the rat. Fertil Steril. 1995; 63:1101–1107.
56. Cai J, Hong Y, Weng C, Tan C, Imperato-McGinley J, Zhu YS. Androgen stimulates endothelial cell proliferation via an androgen receptor/VEGF/cyclin A-mediated mechanism. Am J Physiol Heart Circ Physiol. 2011; 300:H1210–H1221.
57. Godoy AS, Chung I, Montecinos VP, Buttyan R, Johnson CS, Smith GJ. Role of androgen and vitamin D receptors in endothelial cells from benign and malignant human prostate. Am J Physiol Endocrinol Metab. 2013; 304:E1131–E1139.
58. Ong PJ, Patrizi G, Chong WC, Webb CM, Hayward CS, Collins P. Testosterone enhances flow-mediated brachial artery reactivity in men with coronary artery disease. Am J Cardiol. 2000; 85:269–272.
59. Raitakari OT, Celermajer DS. Flow-mediated dilatation. Br J Clin Pharmacol. 2000; 50:397–404.
60. Tarutani Y, Matsumoto T, Takashima H, Yamane T, Horie M. Brachial artery flow-mediated vasodilation is correlated with coronary vasomotor and fibrinolytic responses induced by bradykinin. Hypertens Res. 2005; 28:59–66.
61. Kang SM, Jang Y, Kim Ji, Chung N, Cho SY, Chae JS, et al. Effect of oral administration of testosterone on brachial arterial vasoreactivity in men with coronary artery disease. Am J Cardiol. 2002; 89:862–864.
62. Empen K, Lorbeer R, Dorr M, Haring R, Nauck M, Glaser S, et al. Association of testosterone levels with endothelial function in men: results from a population-based study. Arterioscler Thromb Vasc Biol. 2012; 32:481–486.
63. Perusquía M, Stallone JN. Do androgens play a beneficial role in the regulation of vascular tone? Nongenomic vascular effects of testosterone metabolites. Am J Physiol Heart Circ Physiol. 2010; 298:H1301–H1307.
64. Eisermann K, Broderick CJ, Bazarov A, Moazam MM, Fraizer GC. Androgen up-regulates vascular endothelial growth factor expression in prostate cancer cells via an Sp1 binding site. Mol Cancer. 2013; 12:7.
65. Fadini GP, Albiero M, Cignarella A, Bolego C, Pinna C, Boscaro E, et al. Effects of androgens on endothelial progenitor cells in vitro and in vivo. Clin Sci (Lond). 2009; 117:355–364.
66. Foresta C, Zuccarello D, De Toni L, Garolla A, Caretta N, Ferlin A. Androgens stimulate endothelial progenitor cells through an androgen receptor-mediated pathway. Clin Endocrinol (Oxf). 2008; 68:284–289.
67. Di Mambro A, Ferlin A, De Toni L, Selice R, Caretta N, Foresta C. Endothelial progenitor cells as a new cardiovascular risk factor in Klinefelter's syndrome. Mol Hum Reprod. 2010; 16:411–417.
68. Foresta C, Caretta N, Lana A, Cabrelle A, Palu G, Ferlin A. Circulating endothelial progenitor cells in subjects with erectile dysfunction. Int J Impot Res. 2005; 17:288–290.
69. Liao CH, Wu YN, Lin FY, Tsai WK, Liu SP, Chiang HS. Testosterone replacement therapy can increase circulating endothelial progenitor cell number in men with late onset hypogonadism. Andrology. 2013; 1:563–569.
70. Cakir E, Ozcan O, Yaman H, Akgul EO, Bilgi C, Erbil MK, et al. Elevated plasma concentration of asymmetric dimethylarginine that is reduced by single dose testosterone administration in idiopathic hypogonadotropic hypogonadism patients. J Clin Endocrinol Metab. 2005; 90:1651–1654.
71. Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001; 108:391–397.
72. Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, et al. HMG-CoA reductase inhibitor mobilizes bone marrow: derived endothelial progenitor cells. J Clin Invest. 2001; 108:399–405.
73. Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005; 26:833–876.
74. Garcia-Cruz E, Leibar-Tamayo A, Romero J, Piqueras M, Luque P, Cardenosa O, et al. Metabolic syndrome in men with low testosterone levels: relationship with cardiovascular risk factors and comorbidities and with erectile dysfunction. J Sex Med. 2013; 10:2529–2538.
75. Camacho EM, Huhtaniemi IT, O'Neill TW, Finn JD, Pye SR, Lee DM, et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013; 168:445–455.
76. Shi Z, Araujo AB, Martin S, O'Loughlin P, Wittert GA. Longitudinal changes in testosterone over five years in community-dwelling men. J Clin Endocrinol Metab. 2013; 98:3289–3297.
77. Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013; 217:R25–R45.
78. Rao PM, Kelly DM, Jones TH. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol. 2013; 9:479–493.
79. Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. Am J Med. 2011; 124:578–587.
80. Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, et al. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003; 107:3059–3065.
81. Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011; 96:3007–3019.
82. Yassin AA, Saad F, Traish A. Testosterone undecanoate restores erectile function in a subset of patients with venous leakage: a series of case reports. J Sex Med. 2006; 3:727–735.
83. Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994; 151:54–61.
84. Zitzmann M, Brune M, Nieschlag E. Vascular reactivity in hypogonadal men is reduced by androgen substitution. J Clin Endocrinol Metab. 2002; 87:5030–5037.
85. Morgentaler A, Sigman M, Miner M. Erectile dysfunction: a precursor to cardiovascular disease. Sex Reprod Menopause. 2009; 7:26–32.
86. Bocchio M, Desideri G, Scarpelli P, Necozione S, Properzi G, Spartera C, et al. Endothelial cell activation in men with erectile dysfunction without cardiovascular risk factors and overt vascular damage. J Urol. 2004; 171:1601–1604.
87. Ribeiro F, Ribeiro IP, Alves AJ, do Ceu Monteiro M, Oliveira NL, Oliveira J, et al. Effects of exercise training on endothelial progenitor cells in cardiovascular disease: a systematic review. Am J Phys Med Rehabil. 2013; 92:1020–1030.
88. Goglia L, Tosi V, Sanchez AM, Flamini MI, Fu XD, Zullino S, et al. Endothelial regulation of eNOS, PAI-1 and t-PA by testosterone and dihydrotestosterone in vitro and in vivo. Mol Hum Reprod. 2010; 16:761–769.
89. Yu J, Akishita M, Eto M, Ogawa S, Son BK, Kato S, et al. Androgen receptor-dependent activation of endothelial nitric oxide synthase in vascular endothelial cells: role of phosphatidylinositol 3-kinase/akt pathway. Endocrinology. 2010; 151:1822–1828.
90. Hirase T, Node K. Endothelial dysfunction as a cellular mechanism for vascular failure. Am J Physiol Heart Circ Physiol. 2012; 302:H499–H505.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download