Abstract
Purpose
To evaluate the recovery of continence after robot-assisted laparoscopic prostatectomy (RALP) and open radical retropubic prostatectomy (RRP).
Materials and Methods
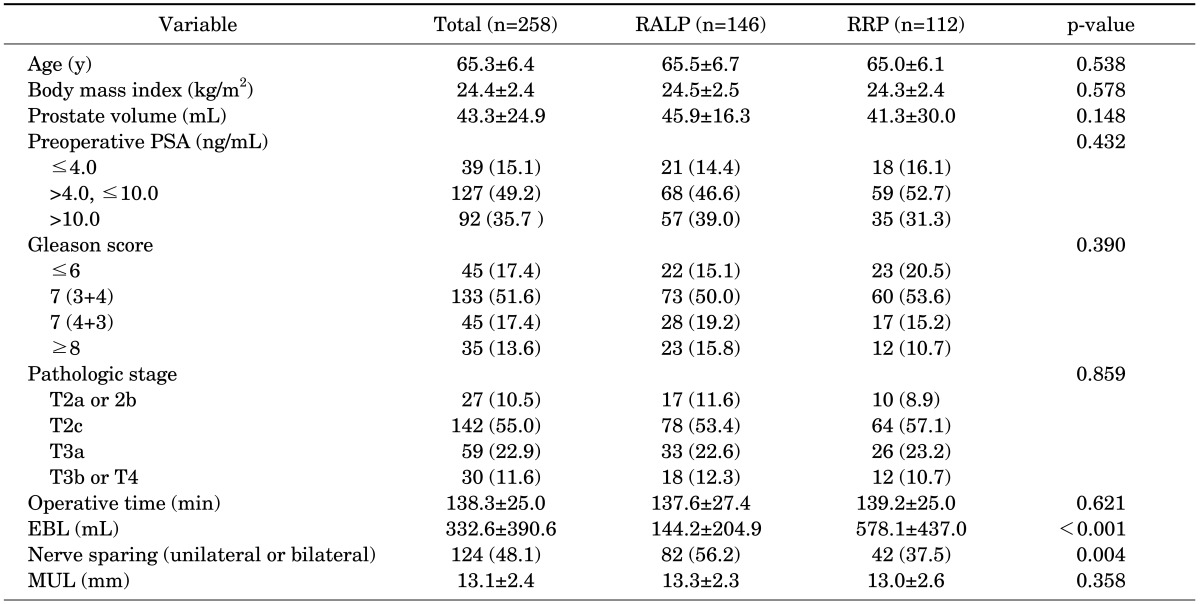
We identified 258 patients who underwent surgery by a single surgeon to treat localized prostate cancer. The patients were divided into two groups according to operative method. In group 1, 146 consecutive patients underwent RALP, and in group 2, 112 patients underwent RRP. To compare the interval until the return of urinary continence between the two groups, we used the Kaplan-Meier method and the log-rank test and Cox proportional hazard regression analyses.
Results
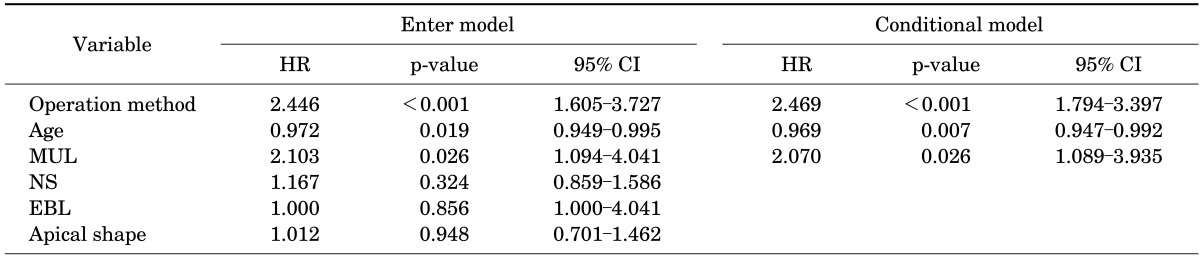
Differences between the two groups were found in mean estimated blood loss (EBL; p<0.001) and the rate of nerve sparing (p=0.004). When continence was defined as the use of 0 to 1 pad per day, 100% of group 1 and 98.2% of group 2 reported continence at 12 months (p=0.189). When continence was defined as no pad use, however, there was a significant difference between the two groups at 12 months: group 1, 95.7%, and group 2, 70.7% (p<0.001). The factors affecting time until no pad use in the univariate analysis with a Cox proportional hazards model were operation method, age, neurovascular bundle saving, membranous urethral length (MUL), EBL, and apical shape. In the multivariate analysis, only operation method, age, and MUL retained significance.
Prostate cancer is the most commonly diagnosed cancer and the second leading cause of cancer-related death in industrialized countries. In Korea, the incidence of prostate cancer is fifth among cancers and is the most rapidly increasing [1]. Radical retropubic prostatectomy (RRP), as described by Walsh [2] in 1982, has been most frequently used to treat localized prostate cancer in the past two decades.
In early 2000, the first robot-assisted laparoscopic radical prostatectomy (RALP) was performed by use of the da Vinci surgical system (Intuitive Surgical Inc., Sunnyvale, CA, USA) in Frankfurt by Binder and Kramer [3]. Through advanced technologies that provide a three-dimensional operative view and laparoscopic instruments that mimic the movement of the human wrist and hand, RALP has reduced the operation time compared with laparoscopic radical prostatectomy.
At present, RRP, the standard treatment modality of localized prostate cancer, has been replaced with RALP because of its minimal invasiveness, reduction in estimated blood loss (EBL), shorter hospital stay, and reduced postoperative pain. However, few comparative studies have evaluated functional outcomes, such as potency and continence, between RRP and RALP. The prevalence of incontinence after radical prostatectomy varies from 2.5% to 87% depending on the definition of urinary control and the evaluation time [4-9].
The purpose of our study was to compare the time until postoperative continence recovery and to evaluate the risk factors for postoperative incontinence in patients who underwent RRP or RALP by a single experienced surgeon.
The da Vinci surgical system was introduced to Seoul National University Bundang Hospital in October 2007. The clinical data of the patients who underwent surgery to treat localized prostate cancer by a single surgeon were retrieved from a prospectively registered database and the data were reviewed retrospectively. Group 1 consisted of 150 consecutive patients who underwent RALP during the period of July 2008 through June 2009 after the initial 100 cases of RALP to avoid the effect of the learning curve. Group 2 consisted of 150 patients who underwent RRP just before the introduction of the surgical robot in our hospital to minimize the selection bias during the period of September 2006 through October 2007. A total of 42 cases were excluded because of missing data; accordingly, a total of 258 cases (group 1, n=146; group 2, n=112) were included in the final analysis.
All RRP and RALP procedures were performed by a single experienced surgeon (S.E.L). RRP was performed in the manner of a conventional operation without periurethral reconstruction. RALP was done in the manner of the conventional transperitoneal approach by use of the four-armed da Vinci surgical robot system. A six-port technique described elsewhere [10] was adopted with minor modifications with anterior reconstruction for incontinence [11]. In both groups, unilateral or bilateral neurovascular bundle saving when indicated was done by use of the athermal technique. Pelvic lymph node dissection was performed in men with a high risk of cancer at the discretion of the surgeon. None of the patients involved in the study received preoperative radiation therapy or androgen-deprivation therapy. A total of 258 cases (146 for group 1 and 112 for group 2), excluding 42 cases missing information on continence or basic information, were included in the final analysis.
We used the Kaplan-Meier method and the log-rank test to analyze the differences between the curves to compare the interval until the return of urinary continence between the two groups. The difference in the continence condition of the two groups at 12 months after the operation was confirmed by use of the chi-square test and Student t-test. We performed univariate Cox proportional hazard regression analyses to assess the recovery of continence. All significant variables in the univariate analyses were included in the multivariate analyses with enter model at the initial steps. Subsequently, multivariate Cox regression models with backward elimination with the probability for removal of 0.1 were obtained. All statistical analyses were performed by use of PASW ver. 17.0 (SPSS Inc., Chicago, IL, USA). For all statistical comparisons, differences with p<0.05 were considered statistically significant.
We evaluated continence status at 2 weeks and 1, 3, 6, 9, and 12 months postoperatively at an outpatient office. Continence was evaluated by using question 5 of the Expanded Prostate Cancer Index Composite questionnaire ("How many pads or adult diapers per day did you usually use to control leakage during the last 4 weeks?"). Continence was defined as no pad use. However, pad use of 0 to 1 pad per day was also evaluated to allow comparisons with previous studies.
The length of the membranous urethra was measured on the magnetic resonance imaging (MRI) T2-weighted coronal images as the distance from the prostatic apex to the entry of the urethra into the penile bulb as previously described [12,13]. The prostatic apex shape was evaluated on the MRI midsagittal images. The shape was classified as either "no overlapping between apex and membranous urethra" or "anterior and/or posterior overlapping between apex and membranous urethra" according to the results of a previous study [12].
The mean age of all patients was 65.3±6.4 years, and the mean preoperative prostate-specific antigen concentration was 13.0±20.4 ng/mL. Differences were found between the two patient groups in mean EBL (144.2±204.9 mL vs. 578.1±437.0 mL, p<0.001) and the rate of nerve sparing (56.2% vs. 37.5 %, p=0.004) (Table 1).
Kaplan-Meier curves demonstrated that when continence was defined as a requirement for no pads or a single secure pad per day, the continent rate was significantly higher in group 1 than in group 2 (log-rank test, p=0.008). Also, when continence was defined as no pad use, the continent rate was significantly higher in group 1 than in group 2 (log-rank test, p<0.001). When continence was defined as pad use of 0 to 1 pad per day, 100% of group 1 and 98.2% of group 2 reported continence at 12 months (chi-square test, p=0.189). When continence was defined as no pad use, however, there was a significant difference between the two groups at 12 months: group 1, 95.7%, and group 2, 70.7% (chi-square test, p<0.001) (Fig. 1).
We performed univariate Cox proportional hazard regression analyses to assess for contributing factors to continence recovery. In the univariate analysis, operation method (p<0.001; hazard ratio [HR], 2.493; 95% confidence interval [CI], 1.820 to 3.414), age (p=0.012; HR, 0.971; 95% CI, 0.949 to 0.994), neurovascular bundle saving (bilateral or unilateral: p=0.008; HR, 1.474; 95% CI, 1.108 to 1.961), membranous urethral length (MUL: p=0.009; HR, 2.224; 95% CI, 1.221 to 4.052), EBL (p=0.003; HR, 0.999; 95% CI, 0.999 to 1.000), and apical shape (overlapping vs. nonoverlapping: p=0.025; HR, 1.483; 95% CI, 1.049 to 2.095) were statistically significant. In the multivariate analysis, however, only operation method, age, and MUL retained statistical significance (Table 2).
At present, RRP is considered a standard treatment modality for localized prostate cancer. Despite technological progress, however, some morbidity related to surgery, such as postoperative incontinence, remains and is the same as in the case of minimally invasive surgery. Patient factors such as age, body mass index, and prostate gland volume and surgical factors such as bladder neck-sparing technique, neurovascular bundle preservation, puboprostatic ligament preservation, dorsal vein complex control technique, and maximizing urethral length have been implicated in urinary control after radical prostatectomy but without consistent results.
Since RALP was introduced, Ahlering et al. [14] reported the recovery rates of postoperative incontinence at 3 months for RRP and RALP as 76% vs. 75% (p=not significant), respectively. Di Pierro et al. [15] reported postoperative incontinence recovery at 3 months as 95% for RALP and 83% for RRP (p=0.003) and that after 12 months as 89% for RALP and 80% for RRP (p=0.92). Also, Krambeck et al. [16] reported that there was no significant difference in postoperative continence between RALP (91.8%) and RRP (93.7%) (p=0.344) at 1 year. However, Ficarra et al. [17] reported opposite results of postoperative incontinence for RRP and RALP as 41% vs. 81% after catheter removal (p<0.001) and 88% vs. 97% at 12 months (p=0.01), respectively. Tewari et al. [18] also reported the duration to 50% recovery of postoperative incontinence as 44 days for RALP and 160 days for RRP. These studies used the definition of continence as the use of 0 to 1 pad compared with no pad. But there is no consensus on the definition of continence, and there is movement to standardize the definition of continence as a satisfied, pad-free state. Krupski et al. [5] and Olsson et al. [6] reported that 30% to 55% of patients with no pad use after surgery still intermittently suffer from incontinence. Herein, our study compared the continence state as both no pad and 0 to 1 pad to overcome the problem of previous studies. If the studies by Ahlering et al. [14] and Di Pierro et al. [15] had defined continence strictly as the no pad state, the difference in postoperative incontinence would be intensified.
Paparel et al. [19] reported that MUL before or after surgery has a significant effect on postoperative continence statistically (p<0.01). Those authors reported a close correlation among the amount of MUL loss and postoperative continence (p=0.02) and periurethral fibrosis. Minimizing trauma during the operation to save MUL by correct exfoliation of the prostate apex had an impact on continence [19]. Lee et al. [12] reported a similar result. The results of this study verify that MUL is a significant factor for continence recovery.
In our study, RRP was performed 2 years earlier than RALP. To eliminate selection bias and to minimize the possible learning curve, the RRP group was selected from patients who had undergone RRP just before the 150 consecutive RALP patients.
Our results suggest that advantages for continence recovery may be present in those patients undergoing RALP compared with RRP. The robotic system used in RALP provides a magnified three-dimensional view and precise articulation instruments. These benefits, in terms of urethrovesical anastomosis and anterior reconstruction, might contribute to the early recovery of urinary continence.
The strengths of our study are that the surgery was performed by a single experienced surgeon with uniform surgical technique and we used a validated self-administered questionnaire. To date, some articles have compared oncological and functional outcomes between RALP and RRP, but few comparisons have been made of procedures performed by a single surgeon at a single institute. The comparison that Tewari et al. [18] made in 2003 with 100 cases of RRP and 200 cases of RALP was of procedures performed at a single facility but not by a single surgeon. The comparison that Ficarra et al. [17] made in 2009 between RRP and RALP was also not of procedures performed by a single surgeon. Because a study design with a single surgeon includes the same standards for patient selection and the same postoperative management protocol, such a design has advantages for ruling out the possibility of selection bias and confounding bias.
Our study did have some limitations. First, there was a 2-year gap between the groups for this study. We did not use a quality of life questionnaire for the use of security pads and no pad usage. Finally, the retrospective design may also be a limitation, but we made a concerted effort to complete data collection and minimize bias by designing a prospective database. Therefore, these results should be confirmed by several other surgeons and through well-designed randomized controlled trials. Currently, some randomized controlled trials are in progress, e.g., the laparoscopic prostatectomy robot open study [20].
The results of our study suggest that RALP is an independent factor for the recovery of continence, as were age and MUL, and RALP has advantages for postoperative continence recovery and the quality of continence compared with RRP. This result should be validated by well-conducted prospective randomized controlled trials in the future.
References
1. Won YJ, Sung J, Jung KW, Kong HJ, Park S, Shin HR, et al. Nationwide cancer incidence in Korea, 2003-2005. Cancer Res Treat. 2009; 41:122–131. PMID: 19809561.

2. Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol. 1998; 160(6 Pt 2):2418–2424. PMID: 9817395.
3. Binder J, Kramer W. Robotically-assisted laparoscopic radical prostatectomy. BJU Int. 2001; 87:408–410. PMID: 11251539.

4. Foote J, Yun S, Leach GE. Postprostatectomy incontinence. Pathophysiology, evaluation, and management. Urol Clin North Am. 1991; 18:229–241. PMID: 2017806.
5. Krupski TL, Saigal CS, Litwin MS. Variation in continence and potency by definition. J Urol. 2003; 170(4 Pt 1):1291–1294. PMID: 14501744.

6. Olsson LE, Salomon L, Nadu A, Hoznek A, Cicco A, Saint F, et al. Prospective patient-reported continence after laparoscopic radical prostatectomy. Urology. 2001; 58:570–572. PMID: 11597541.

7. Lepor H, Kaci L. The impact of open radical retropubic prostatectomy on continence and lower urinary tract symptoms: a prospective assessment using validated self-administered outcome instruments. J Urol. 2004; 171:1216–1219. PMID: 14767305.

8. Litwin MS, Lubeck DP, Henning JM, Carroll PR. Differences in urologist and patient assessments of health related quality of life in men with prostate cancer: results of the CaPSURE database. J Urol. 1998; 159:1988–1992. PMID: 9598504.

9. Wei JT, Dunn RL, Marcovich R, Montie JE, Sanda MG. Prospective assessment of patient reported urinary continence after radical prostatectomy. J Urol. 2000; 164(3 Pt 1):744–748. PMID: 10953138.

10. Menon M, Shrivastava A, Kaul S, Badani KK, Fumo M, Bhandari M, et al. Vattikuti Institute prostatectomy: contemporary technique and analysis of results. Eur Urol. 2007; 51:648–657. PMID: 17097214.

11. Tewari AK, Bigelow K, Rao S, Takenaka A, El-Tabi N, Te A, et al. Anatomic restoration technique of continence mechanism and preservation of puboprostatic collar: a novel modification to achieve early urinary continence in men undergoing robotic prostatectomy. Urology. 2007; 69:726–731. PMID: 17445659.

12. Lee SE, Byun SS, Lee HJ, Song SH, Chang IH, Kim YJ, et al. Impact of variations in prostatic apex shape on early recovery of urinary continence after radical retropubic prostatectomy. Urology. 2006; 68:137–141. PMID: 16777192.

13. Coakley FV, Eberhardt S, Kattan MW, Wei DC, Scardino PT, Hricak H. Urinary continence after radical retropubic prostatectomy: relationship with membranous urethral length on preoperative endorectal magnetic resonance imaging. J Urol. 2002; 168:1032–1035. PMID: 12187216.

14. Ahlering TE, Woo D, Eichel L, Lee DI, Edwards R, Skarecky DW. Robot-assisted versus open radical prostatectomy: a comparison of one surgeon's outcomes. Urology. 2004; 63:819–822. PMID: 15134953.

15. Di Pierro GB, Baumeister P, Stucki P, Beatrice J, Danuser H, Mattei A. A prospective trial comparing consecutive series of open retropubic and robot-assisted laparoscopic radical prostatectomy in a centre with a limited caseload. Eur Urol. 2011; 59:1–6. PMID: 21035248.

16. Krambeck AE, DiMarco DS, Rangel LJ, Bergstralh EJ, Myers RP, Blute ML, et al. Radical prostatectomy for prostatic adenocarcinoma: a matched comparison of open retropubic and robot-assisted techniques. BJU Int. 2009; 103:448–453. PMID: 18778350.

17. Ficarra V, Novara G, Fracalanza S, D'Elia C, Secco S, Iafrate M, et al. A prospective, non-randomized trial comparing robot-assisted laparoscopic and retropubic radical prostatectomy in one European institution. BJU Int. 2009; 104:534–539. PMID: 19281468.

18. Tewari A, Srivasatava A, Menon M. Members of the VIP Team. A prospective comparison of radical retropubic and robot-assisted prostatectomy: experience in one institution. BJU Int. 2003; 92:205–210. PMID: 12887468.

19. Paparel P, Akin O, Sandhu JS, Otero JR, Serio AM, Scardino PT, et al. Recovery of urinary continence after radical prostatectomy: association with urethral length and urethral fibrosis measured by preoperative and postoperative endorectal magnetic resonance imaging. Eur Urol. 2009; 55:629–637. PMID: 18801612.

20. Thorsteinsdottir T, Stranne J, Carlsson S, Anderberg B, Bjoholt I, Damber JE, et al. LAPPRO: a prospective multicentre comparative study of robot-assisted laparoscopic and retropubic radical prostatectomy for prostate cancer. Scand J Urol Nephrol. 2011; 45:102–112. PMID: 21114378.

FIG. 1
Continent rate after surgery. (A) When continence was defined as no pad or a single secure pad requirement per day. (B) When continence was defined as no pad use. RALP, robot-assisted laparoscopic prostatectomy; RRP, radical retropubic prostatectomy.
*Chi-square test.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download