Abstract
Purpose
We report our experience with laparoendoscopic single-site (LESS) urological procedures in children less than 5 years of age.
Materials and Methods
Ten patients (11 procedures) underwent LESS through the umbilicus. Seven patients underwent nephrectomy and three patients underwent pyeloplasty (one simultaneous bilateral). R-port port (Advanced Surgical Concepts, Ireland) was used in nine cases, in one case, the Gelpoint access port (Applied Medical, Rancho Santa Margarita, CA, USA) was used. The Olympus Endoeye camera with coaxial light cable was used. The hilum was secured in all cases with Hem-o-Lok clips (Teleflex Medical, Research Triangle Park, NC, USA) except in one case in which an Endo GIA stapler (Covidien Surgical, Norwalk, CT, USA) was used.
Results
All procedures were technically successful. Accessory port (3 mm) was used in 3 patients. Mean age in nephrectomized patients was 3.14±1.7 years, the mean operative room time (ORT) was 97.5±12.54 minutes. In the pyeloplasty group, mean ORT was 192±47.16 minutes and mean age was 2.43±2.3 years. Bilateral pyeloplasty was done in a 4-month-old infant. The ORT in this case was 180 minutes. A follow-up renogram done in the pyeloplasty patients (n=2) showed good drainage. Mean length of stay was 3.6 days (range, 3 to 6 days).The analgesic requirement was 23.86 mg (range, 12.5 to 50 mg) of diclofenac sodium.
Laparoendoscopic single-site surgery (LESS) in urology was first described by Rane et al. [1]. The LESS approach has been utilized for various indications in adults, including donor nephrectomy, simple nephrectomy, and reconstructions such ureteral reimplantation and pyeloplasty. The safety and efficacy of the standard laparoscopic approach has also been described in pediatric populations, in which studies have compared outcomes between the laparoscopic and open approaches [2,3]. However, there is a paucity of literature regarding application of the LESS approach in children. Concerns regarding the application of LESS in children are the smaller working space and the need for specialized instrumentation. In this article, we review our experience with LESS in children. We detail the intraoperative outcomes and the measures taken to reduce the level of difficulty.
This was a retrospective analysis of all patients younger than 5 years who underwent LESS between July 2010 and June 2012. Prior approval of the institutional review board was obtained. The parameters analyzed included operating room time, blood loss, hospital stay, and complications.
All procedures were performed with the patient in a position similar to that used for standard laparoscopy. The patient was placed at the edge of the table with the arms padded and secured. It was made certain that no tubes (suction, irrigation) were placed on the torso. The eyes were padded and taped. The patients were secured with the help of tape; however, the use of a bean bag would also be suitable. The surgeon stood while the assistant (camera driver) sat to make space in the operating area. This made the procedure ergonomically less challenging.
All procedures were done by use of the R-port multi-instrument access port (Advanced Surgical Concepts, Bray, Ireland), except for one in which a Gelpoint port (Applied Medical, Rancho Santa Margarita, CA, USA) was used. The R-port is available as either a Triport or a Quadport. We feel the Triport variant is more suitable for the pediatric age group. The ports are single-use and have one 10-mm port channel and two 5-mm channels for instrument access. The port features a plastic sleeve that connects two rings, one abdominal and one peritoneal. The ports were inserted by using the open technique. An umbilical skin crease incision was made and the fascia incised. The skin incision was hidden in the umbilical skin crease. The key to proper insertion is an optimal size of the facial opening. The opening should not be too large, because the port tends to slip out; neither should it be too small, because this may cause difficulty in introduction of both the port and the instruments. A larger facial incision causes gas leaks during surgery. Once the port was inserted, the plastic sleeve was pulled down so that the plastic rings (abdominal and peritoneal) approximated and the port fit snugly on the abdominal wall. In three of our cases, an accessory port was used. The common reasons for introduction of accessory ports included difficulty in upper pole dissection, inadequate exposure of the renal hilum, and difficulty in suturing in reconstructive procedures. The Gelpoint mini port accommodates various abdominal wall and incision sizes, provides continuous access, and ensures improved articulation of both 5-mm and 10-mm instruments.
The choice of instruments is a matter of surgeon preference; the surgeon should choose instruments he is familiar with. Nonarticulating straight laparoscopic instruments were used in all our cases. The nephrectomy followed the typical steps of dissection of the ureterogonadal packets followed by dissection of the hilum (Fig. 1). In all but one case, the hilum was secured by using Hem-o-Lok clips (Teleflex Medical, Research Triangle Park, NC, USA) with two clips placed on the patient side. In one case, an Endo GIA stapler (Covidien Surgical, Norwalk, CT, USA) was placed through a 10-mm port inserted through the Gelpoint port. The LESS approach affords the advantage of retrieval of the specimen from the port itself without the need to extend the incision (Figs. 1, 2).
Before a pyeloplasty in pediatric patients, we preferred to place an ultrasound-guided antegrade ureteral catheter. Before placing the patient in the laparoscopy position, an ultrasound-guided percutaneous access was gained, and two guide wires were placed in the pelvicaliceal system. Over one guide wire a nephrostomy was inserted, whereas over another the ureteral catheter was coiled in the renal pelvis. The advantages of this arrangement are that the ureteral catheter apart from being used as a splint can also be used as a conduit for passage of the stent. In addition, as a protocol, we removed the ureteral catheter first, followed by the nephrostomy. This helped to ensure the patency of the anastomosis if a problem was contemplated. The antegrade ureteral catheter and nephrostomy help to avoid the urethral route for stent removal. The Salle stent works on a similar principle except that it is inserted intraoperatively and has a flower at its end that is self-retaining. Intraoperatively during dissection, the nephrostomy was clamped, which distends the pelvis and facilitated dissection.
All pyeloplasties were done with a modified Anderson technique, and the pelvis in all cases was extrarenal and required reduction. The pelvis was hitched up by using a suture placed strategically through the abdominal wall. This helped in dissection of the pelviureteral junction and avoided the need to place an extra port. The suture helped in orienting the pelvis during suturing.
We performed a bilateral simultaneous LESS pyeloplasty in a 4-month-old child. A Triport access port was used during the procedure. An ultrasound-guided antegrade ureteral splint along with a percutaneous nephrostomy was placed. The right side was dealt with first, followed by the left side. The hitch stitch placed through the abdominal wall was useful. The stitch was placed by using a taper-cut needle on a 3/8th circle needle (Fig. 3). The exact site of entry of the needle was determined under direct laparoscopic vision. The needle was passed through the medial aspect of the renal pelvis. This helped in orienting as well as stabilizing the pelvis during suturing. The antegrade ureteral catheter that acted as a splint was removed on the third postoperative day, and the nephrostomy was clamped for 24 hours and removed.
A total of 11 LESS procedures were performed on 10 patients (1 was bilateral). All procedures were completed successfully. An accessory port (3 mm) was inserted in 3 patients. In children undergoing nephrectomy (n=7), the mean age was 3.14±1.7 years, and the mean operating room time was 97.5±12.54 minutes. No perioperative complications were encountered.
In the pyeloplasty group, the mean operating room time was 192±47.16 minutes and the mean patient age was 2.43±2.3 years. Bilateral pyeloplasty was done in one patient. The operating room time in this case was 180 minutes. A follow-up renogram at 6 months was available for two patients and showed good drainage. In all our patients who underwent ablative procedures, the specimen could be retrieved without increasing the incision. Mean estimated blood loss was 50 mL. The blood loss was calculated by measuring the output in the suction irrigation chamber. Mean length of stay was 3.6 days (range, 3 to 6 days). The patient characteristics are as detailed in Table 1.
Since the first description of LESS surgery in 2007 [1], various surgical procedures have been performed with this approach in urology and allied surgical fields. The literature is scant regarding the application of this approach in small patients, however. As with any surgical procedure, LESS involves a learning curve. The advantages of LESS in the pediatric age group are well-defined tissue planes, absence of fat, and thin abdominal walls. These characteristics of the pediatric age group help in easier dissection of surgical planes. The challenges in development of LESS revolve around technique and anesthesia-related issues.
From a technical standpoint, the challenges include a need for specialized instrumentation and loss of triangulation. Although a few workers have used articulating and bent instruments, we used standard instruments in our series. The technical challenges one encounters are in-line camera and instrument angles and a need for coordination with an experienced camera driver. The smaller working space in infants and toddlers also increases the level of difficulty.
The various maneuvers that we have used to reduce instrument clashing are as follows: 1) Camera driver position: The camera driver sits behind the surgeon, which allows the operating surgeon increased space for movement and decreases crowding in the surgical field.
2) Use of long and short instruments in either hand: The instrument can be an articulating or a nonarticulating instrument. This instrument arrangement helps in improving the triangulation.
3) Hand and instrument crossover: This is useful during dissection of the upper pole, because this is one of the most challenging steps in the procedure.
4) Use of a hitch stitch in reconstructive procedures: Among the various maneuvers one can use to avoid an extra port is the use of a transabdominal hitch stitch. This stitch is passed through the abdominal wall and is useful in reconstructive procedures such as pyeloplasty. A similar stitch has also been described for lifting the uretrogonadal packet while performing LESS nephrectomy.
5) Use of a long suction and a Harmonic scalpel: We do not use articulating or prebent instruments; however, an extra-long suction is useful during dissection of the upper pole. This is of significance in adults and older children. The extra-long suction helps to take down the lienorenal or Splenorenal ligament. This part of the dissection is essential for exposure of the renal hilum.
6) Use of a camera with coaxial light cable: A camera with a coaxial light cable helps in decreasing instrument clashing; alternatively, a flexible tip camera helps in providing the surgeon a vantage of the operating field without causing clashing.
7) Use of an accessory port: It is debatable whether adding an extra port defeats the purpose of performing the procedure through a single incision.
Suturing is one of the most challenging steps in LESS. The steps particularly relevant to facilitating suturing are, first, the use of one straight instrument and one bent instrument (to improve triangulation); second, the use of a hitch stich for retraction of the redundant pelvis and orienting the anatomy during the anastomosis; and third, the use of an extra 3-mm port to facilitate suturing.
We prefer to use the Triport variant of the R-port. There are reports describing pediatric LESS in children using the SILSTM (Covidien, Norwalk, CT, USA) port [4]. From an anesthesia perspective, the pneumoperitoneum pressures should be kept at 8 to 10 mm of Hg. It is the responsibility of the anesthetist and the surgeon to ensure that the child is positioned properly on the table. It should be ensured that the access port fits snugly, thus preventing subcutaneous emphysema. In addition, the pneumoperitoneum pressures should be kept at 8-10, and all efforts made to prevent hypothermia.
LESS has the potential advantage of improved cosmesis. It has been argued that LESS with a scar hidden in the umbilicus is cosmetically superior than a scar in the adjacent abdominal wall. In the pediatric age group it has been proved that laparoscopy provides better outcomes with better cosmesis [5]. Evidence suggests that visible scarring in children reduces their self-esteem, resulting in impaired socialization skills. Thus, lesser scarring through the umbilical incision offered with LESS will have a lower psychosocial impact on the growing child [6].
We did not encounter any intraoperative or postoperative complications. The overall complication rate for pediatric urologic laparoscopy is small [7]. It is known that infants have a greater risk of port site hernias; however, we did not notice any defects in the scars on follow-up in any of our cases [8]. In the series by Koh et al. [9] two patients developed unilateral hydroceles in the first postoperative week.
In the pediatric population, the relatively less fat and thin musculature make dissection easy and facilitate easy port insertion. The characteristics of the abdominal wall also make retrieval of specimens easy without the need to extend the incision [9]. Advances in instrumentation that may be beneficial for pediatric patients include the Spider surgical system (Transenterix, Durham, NC, USA). This is a single-access device that has two flexible channels that are inserted in the abdomen [10]. This has the potential to improve triangulation. The use of magnets for retraction has been described in adults; however, their utility in children has yet to be proven [11]. The shortcomings and difficulties in development of LESS in the pediatric age group include the lack of adequately powered randomized studies comparing LESS with conventional laparoscopy in various procedures. Patient safety is the prime consideration, because in children there is no room for error. There is a need to develop simulators in the future for LESS training in the pediatric age group. This will generate enthusiasm among pediatric urologists to develop the technique. The major hindrance in the development of LESS is the lack of appropriate instrumentation. U. S. Food and Drug Administration data show that the development of pediatric medical devices lags by 2 years compared with adult equipment [12]. A significant reason is the small patient population, which hinders research and development.
Although a head-on comparison between standard laparoscopy and LESS in a pediatric population was beyond the scope of this study, we have eluded to our anecdotal experience with standard laparoscopic pyeloplasty in children [13]. The results of LESS match those of standard pyeloplasty done at our center. We do acknowledge the shortcomings of our study. These include the small sample size. Furthermore, the study was noncomparative and lacked objective data on cosmesis. The real advantages of LESS in children in terms of cosmesis and the perceived "smaller" length of incision need to be proven in larger randomized controlled trials.
The results from our series show that LESS is technically feasible in small patients as young as 4 months of age. Our initial experience suggests that LESS offers better cosmesis. This needs to be proved objectively through comparative studies. The development of smaller instruments will allow further growth of this technique in this patient subgroup.
Figures and Tables
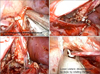 | FIG. 1The steps of laparoendoscopic single-site nephrectomy mirror those of standard multiport laparoscopy. IVC, inferior vena cava. |
References
1. Rane A, Rao P, Rao P. Single-port-access nephrectomy and other laparoscopic urologic procedures using a novel laparoscopic port (R-port). Urology. 2008. 72:260–263.
2. Hamilton BD, Gatti JM, Cartwright PC, Snow BW. Comparison of laparoscopic versus open nephrectomy in the pediatric population. J Urol. 2000. 163:937–939.
3. Raman JD, Bagrodia A, Cadeddu JA. Single-incision, umbilical laparoscopic versus conventional laparoscopic nephrectomy: a comparison of perioperative outcomes and short-term measures of convalescence. Eur Urol. 2009. 55:1198–1204.
4. Tugcu V, Ilbey YO, Polat H, Tasci AI. Early experience with laparoendoscopic single-site pyeloplasty in children. J Pediatr Urol. 2011. 7:187–191.
5. Piaggio LA, Franc-Guimond J, Noh PH, Wehry M, Figueroa TE, Barthold J, et al. Transperitoneal laparoscopic pyeloplasty for primary repair of ureteropelvic junction obstruction in infants and children: comparison with open surgery. J Urol. 2007. 178(4 Pt 2):1579–1583.
6. Broder HL, Smith FB, Strauss RP. Effects of visible and invisible orofacial defects on self-perception and adjustment across developmental eras and gender. Cleft Palate Craniofac J. 1994. 31:429–436.
7. Peters CA. Complications in pediatric urological laparoscopy: results of a survey. J Urol. 1996. 155:1070–1073.
8. Cost NG, Lee J, Snodgrass WT, Harrison CB, Wilcox DT, Baker LA. Hernia after pediatric urological laparoscopy. J Urol. 2010. 183:1163–1167.
9. Koh CJ, De Filippo RE, Chang AY, Hardy BE, Berger A, Eisenberg M, et al. Laparoendoscopic single-site nephrectomy in pediatric patients: initial clinical series of infants to adolescents. Urology. 2010. 76:1457–1461.
10. Ponsky TA, Krpata DM. Single-port laparoscopy: considerations in children. J Minim Access Surg. 2011. 7:96–98.
11. Cadeddu J, Fernandez R, Desai M, Bergs R, Tracy C, Tang SJ, et al. Novel magnetically guided intra-abdominal camera to facilitate laparoendoscopic single-site surgery: initial human experience. Surg Endosc. 2009. 23:1894–1899.
12. National Institutes of Health. Pediatric device consortia grant program (P50). Request for applications (RFA) number: RFA-FD-09-007 [Internet]. c2013. 2013 Feb 26. Bethesda: National Institutes of Health;Available from: http://grants.nih.gov/grants/guide/rfa-files/RFA-FD-09-007.html.
13. Singh H, Ganpule A, Malhotra V, Manohar T, Muthu V, Desai M. Transperitoneal laparoscopic pyeloplasty in children. J Endourol. 2007. 21:1461–1466.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download