Abstract
Purpose
In patients with neurogenic bladder due to spinal cord injury or disease who undergo augmentation cystoplasty (AC) for not only bladder dysfunction but also sphincteric incontinence, the need for concomitant bladder neck reconstruction at the time of AC has not yet been established. The aim of this study was to evaluate whether concomitant bladder neck reconstruction is necessary when performing AC.
Materials and Methods
We retrospectively investigated 35 patients who underwent AC from January 2006 to September 2010. Medical history, preoperative and postoperative fluoroscopic urodynamic study (FUDS) parameters, and responses to an incontinence questionnaire (ICIQ Korean version) were reviewed.
Results
A final analysis was performed on 17 patients (9 male, 8 female) who were diagnosed with sphincteric incontinence. Continence status, the number of pads used, and the bother score were significantly improved postoperatively in this subpopulation. Preoperatively, all patients used pads, and the average daily number was 2.2 (median; range 0 to 6). Postoperatively, the number of pads used decreased significantly to 0.9 (median; range 0 to 3) pads a day (p=0.002). Urodynamic parameters including bladder capacity, compliance, involuntary detrusor contraction, and bladder neck incompetence proven by FUDS were also significantly improved.
Conclusions
Our study demonstrated that both objective urodynamic parameters and subjective incontinence symptoms improved significantly after the completion of AC as a single procedure in patients with sphincteric incompetence. This implies that anti-incontinence bladder outlet surgery does not have to be performed simultaneously and can be considered later as a staged operation.
In patients with neurogenic bladders as the result of spinal cord injury or spinal disease, complications such as upper urinary tract damage, infection, stone formation, and incontinence commonly occur. In such patients, procedures that are intended to decrease bladder pressure are required to prevent further deterioration of the upper tract. Among such procedures, augmentation cystoplasty (AC) is considered to be the gold standard. Incontinence can occur as the result of sphincteric incompetence or bladder factors such as involuntary detrusor contraction (IDC) or poor compliance. In patients with sphincteric incontinence, pharmaceutical agents can be prescribed as a first-line treatment. When the incontinence is refractory to medication, surgical treatment such as slings, artificial urinary sphincters (AUSs), or injection of bulking agents can be considered. In many patients, both sphincteric and bladder-related causes of incontinence coexist. However, consensus over whether concomitant or staged anti-incontinence surgery with AC is advisable in patients with sphincteric incontinence has not yet been established.
The surgical management of neurogenic sphincteric incontinence is still under debate, and an effective method for long-term continence in subjects with neurogenic sphincteric incompetence remains to be found. Stein et al. [1] reported on a concomitant fascial sling procedure performed in a male pediatric patient. This patient underwent AC for neurogenic bladder and bladder neck suspension for his incompetent urinary sphincter with satisfactory results. Also, Chartier-Kastler et al. [2] reported lower complication rates in patients who underwent AC and AUS implantation simultaneously than in patients who underwent staged operations. Dave and Salle [3] analyzed a Kropp-Salle bladder neck repair performed in 9 children, 6 of whom had myelodysplasia. Eighty-nine percent of these patients received concomitant AC, and 88% were dry postoperatively. With concomitant surgery, however, there is a possibility of a higher incidence of morbidities in patients whose incontinence might be resolved with bladder outlet procedures performed as a single procedure.
Selecting the treatment method for patients with neurogenic sphincter incontinence is a challenging clinical issue. With the goal of obtaining maximal surgical efficacy with the minimum inconvenience to patients, we evaluated patients with sphincteric incontinence who underwent AC as a single procedure at our center. We aimed to determine whether concomitant bladder neck reconstruction was necessary when performing AC in patients with neurogenic bladder.
We retrospectively investigated clinical data from 35 patients who underwent AC from January 2006 to September 2010. Medical history, preoperative and postoperative fluoroscopic urodynamic study (FUDS) parameters, and responses to an incontinence questionnaire (ICIQ Korean version) were reviewed. The ICIQ questionnaires were previously validated linguistically by bilingual translators and were approved for use by the International Consultation on Incontinence Modular Questionnaire Advisory Board (http://www.iciq.net/index.html). We analyzed these patients to see whether AC performed as a single procedure could improve their continence status. An ICIQ was obtained at the outpatient clinic postoperatively [4].
Of the patients, 24 with urinary incontinence who filled out both a preoperative and a postoperative ICIQ questionnaire (17 males and 7 females) were eligible for analysis. Of these 24 patients, a final analysis was performed on 17 patients who complained of urinary incontinence preoperatively and showed sphincteric incontinence. Sphincteric incontinence was primarily determined by a clinical decision made on the basis of the presence or absence of incontinence according to the patients' symptoms and signs. It was also supported by a low abdominal leak point pressure (ALPP), combined with low maximal urethral closure pressure (MUCP), and an open bladder neck observed on FUDS [5]. Because the initial group of 35 patients underwent AC, we assumed that all of the patients had bladder factors related to their symptoms.
The FUDS was performed as previously described [6]. Fluoroscopic images were simultaneously obtained, and vesicoureteral reflux, bladder neck competence, and bladder trabeculation were evaluated. Urodynamic parameters including storage phase characteristics, bladder capacity, bladder compliance, cause of incontinence, ALPP, MUCP, maximum cystometric capacity, and detrusor sphincter dyssynergia were evaluated. Fluoroscopic information regarding the bladder neck at filling, trabeculation, and vesico-ureteral reflux (VUR) was also evaluated.
All operations were carried out by one surgeon (SJO) at our institution. The AC procedure was as follows. The patient was placed in a supine position under general anesthesia. A nasogastric tube was inserted, and a Foley urethral catheter was inserted intraoperatively. A lower midline abdominal incision was made and subcutaneous tissue and the anterior rectus sheath was divided. The rectus muscle was retracted laterally, and the posterior rectus sheath and peritoneum were transected. After the native bladder was exposed, a 45-cm portion of the ileum was selected, and according to the patient's condition, the sigmoid colon was used in some cases. The mesentery was divided carefully according to the blood supply. Ileal end-to-end anastomosis was done by use of 3-0 Vicryl. The harvested ileal segment was detubularized along the antimesenteric border. The native bladder was dissected and bivalved vertically from the anterodome to the posterodome area. The detubularized ileal segment was sutured with the bivalved bladder by use of 2-0 Vicryl. A 20 Fr suprapubic cystostomy tube and 2 Jackson-Pratt drains were inserted. Afterward, routine wound closure and dressing were performed.
A descriptive analysis was performed with the independent t-test method. A 2-sided chi-square test was performed by use of a commercial analysis program PASW ver. 18.0 (IBM Co., Armonk, NY, USA). A p-value lower than 0.05 was considered to be statistically significant. This study was approved by the Institutional Review Board of Seoul National University Hospital (number 1106-091-366).
A total of 17 patients with preoperative sphincteric urinary incontinence were analyzed. The demographics of these 17 patients were as follows. The average age of the patients was 34.3 years (standard deviation [SD], ±20.76). Nine patients were male, and 8 were female. The average follow-up period was 10.1 months (SD, ±3.9), and postoperative FUDS was performed a mean of 7.5 months (SD, ±5.3) postoperatively. In 15 patients (88.2%) the ileum was used, and in 2 patients (11.8%) the sigmoid colon was used. Autonomic dysreflexia was present in 2 patients (11.8%). Preoperative VUR was present in 13 patients (76.5%) (Table 1). Postoperatively, 9 of the patients (52.9%) did not have VUR, 2 had left VUR (11.8%), and 1 had right VUR (5.9%). Among the patients with VUR, all of them were grade 2.
Both preoperative and postoperative urodynamic parameters were compared (Table 2). Among the urodynamic parameters evaluated that were related to the bladder, bladder capacity, bladder compliance, and IDC showed significant improvement postoperatively (p<0.05). The bladder neck was incompetent in all patients preoperatively, but was competent in 83.3% (10/12) of the 12 patients whose postoperative FUDS results were available (p=0.004) (Table 3, Fig. 1).
The subjective incontinence status of the patients was evaluated. Concerning the pad number, preoperatively, all patients used pads, and the average daily number was 2.2 (±0.6). Postoperatively, the number of pads used decreased significantly to 0.9 (median; range 0 to 3) pads a day (p=0.002). Significant improvement was shown for questions 1 to 3 on the ICIQ questionnaire as well as for the total score (p<0.05). In the analyzed group, all patients had incon tinence preoperatively, and the number who answered that their incontinence disappeared showed statistically significant improvement (p<0.001). The number of patients who had incontinence when sleeping, those who had nonspecific incontinence, and those who had constant incontinence showed significant improvement (p<0.05). Although postoperatively some patients had incontinence during exercise or when coughing, symptoms disappeared in most patients (Table 4).
In patients with a small or poorly compliant bladder or detrusor overactivity, AC is recommended to lower intravesical pressure and to preserve the upper tract [7]. Although AC can prevent further functional deterioration, resolving incontinence is a pertinent issue. Persistent incontinence after AC deserves attention, because it is an important problem that negatively affects the quality of life of the patients. Borjeson and Lagergren [8] reported incontinence to be the main reason for social isolation of patients with neurogenic bladder. Kaufman et al. [9] and Herschorn and Hewitt [10] reported that 66.6% and 33.8% of patients, respectively, remained incontinent after AC performed as a single procedure. However, in other studies, continence was reported to be achieved after AC as a single procedure. Daher et al. [11] reported improved continence rates after AC performed in a pediatric patient population in a 10-year follow-up study. Only one patient underwent staged bladder neck reconstruction for postoperative incontinence. Quek and Ginsberg [12] also reported improvement of urodynamic parameters after AC as a single procedure. Venn and Mundy [13] reported a postoperative continence rate of 78% of neurogenic bladder patients who underwent AC only.
Some studies support bladder neck closure for continence; however, this can cause deterioration of a pop-off mechanism of the bladder neck. Siracusano et al. [14] reported that in some patients with neurogenic sphincteric incontinence, an AUS can be considered as a treatment option. In pediatric urology, research about the outcome of bladder outlet procedures on children with urinary incontinence with neuropathic bladder dysfunction or structural deficiency is also rare. If concomitant bladder neck surgery is performed, there is concern about unnecessary surgery. Evaluation of small numbers of patients undergoing AC does not always show improved status on postoperative questionnaires, despite what generally would be considered to be a good surgical outcome. In our study, we evaluated the outcome of AC performed as a single procedure on incontinence caused by sphincteric incompetence. Our data suggested that AC as a single procedure improved both subjective continence proven by the Korean ICIQ questionnaire as well as objective continence proven by decreased pad number and fluoroscopically demonstrated bladder neck competence. When selecting patients for analysis in our study, we reviewed the patient's fluoroscopic images, urodynamic findings, and most importantly, the patient's history. We omitted patients whose leaks on FUDS were accompanied by IDCs. Also, patients whose bladder necks were partially open on cystourethroscopy but showed leaks when strong IDCs occurred on filling cystometrograms were also excluded to avoid ambiguity.
The fact that both a preoperative and a postoperative ICIQ evaluation were available in this study is a strong advantage. Although the preoperative ICIQ evaluation was obtained by a postoperative telephone interview, we considered these data reliable. The reason is that although the evaluation was conducted postoperatively, the patients' recollection of the number of pads they used and the degree of distress caused by incontinence was accurate. These patients had suffered from preoperative incontinence for a long period of time, and their memory was still fresh and the information they provided reliable at the time of data acquisition. The significant improvement of ICIQ scores shows that AC as a single procedure improves the subjective symptoms of the patients. When incontinence occurs when a patient coughs or sneezes, we consider the patient to have stress urinary incontinence (SUI). Postoperatively, 3 patients had SUI; however, their ICIQ total scores decreased from 16 (SD, ±6.2) preoperatively to 7.3 (SD, ±6.4) postoperatively. Therefore, we considered their incontinence to be subjectively improved.
In addition, our study had the advantage of both preoperative and postoperative FUDS in 12 of the patients analyzed. FUDS was performed according to a standardized protocol, and therefore an objective comparison without variation was possible. Also, the operations were all performed by a single experienced surgeon, which provided additional strength to the study.
According to our data, VUR persisted after surgery in 3 patients, which could be attributed to the chimney of the Studer-type enterocystoplasty or to excessive filling during the filling cystometrogram. However, the VUR was observable only at maximal capacity on FUDS and upper-tract follow-up imaging on kidney ultrasound or nonenhanced computed tomography scans showed no hydronephrosis. Patients did not suffer from acute pyelonephritis, either, which led us to conclude that the persistent reflux was caused by overfilling. In some patients, postoperative VUR was observable. The reason for postoperative persistence of VUR could be an elevation of abdominal pressure.
We performed AC on 2 patients who had normal bladder capacity but severe IDC who experienced urine leakage whenever there was an IDC on FUDS. Although some patients showed detrusor overactivity postoperatively, the amplitude and duration of the IDCs were negligible.
This study had some limitations. First, it was performed in a retrospective nature, and second, it was a short-term study. Third, the sample size was relatively small. Due to the limited number of patients, further analysis involving the selection criteria of patients who would require bladder neck procedures either concomitantly or in a staged operation was not possible. However, if more data were accumulated, more valuable information could be provided for patients requiring surgery for incontinence. On the basis of the results of this study, we are able to predict that more valuable information can be obtained and that guidelines for the selection of such patients can be established in the future.
Our study demonstrated that both objective urodynamic parameters and subjective incontinence symptoms improved significantly after AC as a single procedure, even though the patients had sphincteric incompetence. This implies that in patients whose incontinence persists after AC, anti-incontinence bladder outlet procedures can be considered as a staged operation.
Figures and Tables
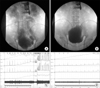
FIG. 1
A 64 year-old male with spinal cord injury showed improvement of bladder compliance and bladder neck competence. His preoperative International Consultation on Incontinence Modular Questionnaire score was 16, and postoperative score was 1. Preoperatively, this patient showed an incompetent bladder neck (A) and poor bladder compliance (C). Six months postoperatively, this patient showed a competent bladder neck (B) and improved bladder compliance (D).
ACKNOWLEDGMENTS
We are obliged to Mr. Kwi-Shik Kim, Mrs. Sang-A Byun, and Ms. Yu Kyung Lee for valuable help in data acquisition.
References
1. Stein R, Wiesner C, Beetz R, Pfitzenmeier J, Schwarz M, Thuroff JW. Urinary diversion in children and adolescents with neurogenic bladder: the Mainz experience. Part II: Continent cutaneous diversion using the Mainz pouch I. Pediatr Nephrol. 2005. 20:926–931.
2. Chartier-Kastler E, Genevois S, Game X, Denys P, Richard F, Leriche A, et al. Treatment of neurogenic male urinary incontinence related to intrinsic sphincter insufficiency with an artificial urinary sphincter: a French retrospective multicentre study. BJU Int. 2011. 107:426–432.
3. Dave S, Salle JL. Current status of bladder neck reconstruction. Curr Opin Urol. 2008. 18:419–424.
4. Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004. 23:322–330.
5. Fleischmann N, Flisser AJ, Blaivas JG, Panagopoulos G. Sphincteric urinary incontinence: relationship of vesical leak point pressure, urethral mobility and severity of incontinence. J Urol. 2003. 169:999–1002.
6. Cho SY, Oh SJ. The clinical significance of rectal contractions that occur during urodynamic studies. Neurourol Urodyn. 2010. 29:418–423.
7. Medel R, Ruarte AC, Herrera M, Castera R, Podesta ML. Urinary continence outcome after augmentation ileocystoplasty as a single surgical procedure in patients with myelodysplasia. J Urol. 2002. 168(4 Pt 2):1849–1852.
8. Borjeson MC, Lagergren J. Life conditions of adolescents with myelomeningocele. Dev Med Child Neurol. 1990. 32:698–706.
9. Kaufman AM, Ritchey ML, Roberts AC, Rudy DC, McGuire EJ. Decreased bladder compliance in patients with myelomeningocele treated with radiological observation. J Urol. 1996. 156:2031–2033.
10. Herschorn S, Hewitt RJ. Patient perspective of long-term outcome of augmentation cystoplasty for neurogenic bladder. Urology. 1998. 52:672–678.
11. Daher P, Zeidan S, Riachy E, Iskandarani F. Bladder augmentation and/or continent urinary diversion: 10-year experience. Eur J Pediatr Surg. 2007. 17:119–123.
12. Quek ML, Ginsberg DA. Long-term urodynamics followup of bladder augmentation for neurogenic bladder. J Urol. 2003. 169:195–198.
13. Venn SN, Mundy AR. Long-term results of augmentation cystoplasty. Eur Urol. 1998. 34:Suppl 1. 40–42.
14. Siracusano S, Ciciliato S, Benedetto PD, Marega D, Tiberio A, Stacul F. An unusual use of AMS 800 artificial urinary sphincter cuff in the treatment of sphincteric neurogenic incontinence: case report. Spinal Cord. 2004. 42:652–654.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download