INTRODUCTION
Adenocarcinoma of the rete testis is a rare tumor. About 70 cases have been reported [1]. The tumor occurs most frequently in elderly males [2]. The primary complaint of most patients with this tumor is testicular swelling [3], which is usually painless. It is not uncommon for this tumor to be of an advanced stage at the time of the diagnosis, and the prognosis is dismal. Although scrotal swelling is the most common manifestation, the clinical presentation is often nonspecific and may not suggest a neoplasm, such that the diagnosis can be delayed [4,5]. To our knowledge, this is the first report in the published literature documenting incidental adenocarcinoma of the rete testis with preceding detection of unilateral hydronephrosis.
CASE REPORT
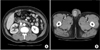
The patient was a 54-year-old man with right flank pain that had started 1 month previously. The kidney-ureter-bladder and urinalysis findings were normal, but ultrasonography showed mild right hydronephrosis. Therefore, abdominopelvic computed tomography (CT) was performed to determine the cause of the hydronephrosis. We found multiple enhanced lesions around the right obturator muscle that were compressing the right distal ureter and causing the hydronephrosis. Multiple retroperitoneal and right common iliac metastatic lymphadenopathies were also seen (Fig. 1A). Additionally, a heterogeneous, low-density lesion with an irregular margin was found with hydrocele in the right testis (Fig. 1B). According to the patient's medical history, the painless swelling of the right scrotum had started about 1 year previously with no other significant medical history. On the physical examination, a relatively movable, solitary hard mass was palpated with swelling. Tumor markers were checked. The lactate dehydrogenase (490 IU/L) and carcinoembryonic antigen (CEA, 40.8 ng/mL) levels were higher than normal, but the alpha-fetoprotein and beta-human chorionic gonadotropin (beta-hCG) levels were normal. A chest X-ray revealed no specific lesions. We decided to perform cystoscopy and retrograde pyelography with radical orchiectomy to rule out any metastatic lesions in the right ureter. A right ureteral stricture was found and a D-J ureteral catheter was inserted. A radical orchiectomy was successfully done.
Grossly, the tumor was centered on the testicular hilum. It was a solid and ill-defined tumor without a capsule. There was no tunica involvement. Microscopic examination showed conventional adenocarcinoma, which shows a glandular pattern of growth with atypical stratified cuboidal to columnar epithelial tumor cells (Fig. 2A). Significant immunohistochemical reactivities for CEA, Muc1, cytokeratin 20, and CDX2 were seen (Fig. 2B). There was no reactivity for prostate-specific antigen (PSA), carbohydrate antigen 19-9, thyroid transcription factor-1, or S-100 protein. There was no neuroendocrine or small cell carcinoma or frank sarcoma component. The resection margins were clear. There were no carcinoma cells in the hydrocele fluid. Positron emission tomography-CT (PET-CT), gastroduodenoscopy, colonoscopy, and transrectal ultrasound were performed in addition to measuring the serum PSA level to rule out metastatic adenocarcinoma from another site. There were no specific findings suggesting other primary lesions. Combined with these findings, we finally diagnosed a right testicular lesion as the primary adenocarcinoma in the rete testis.
After the radical orchiectomy, the patient received combined chemotherapy 6 times, but the progression of the disease was unstoppable. During the course of the chemotherapy, PET-CT was done, which revealed newly developed lung metastasis. The patient died only 8 months after he was first diagnosed with the cancer.
DISCUSSION
The genesis of adenocarcinoma of the rete testis is unknown [6]. This tumor seems to occur most frequently in the seventh decade of life [1]. Up to one quarter of these tumors have been reported in association with scrotal hydrocele [6], as in our case. The diagnosis is often difficult and made incidentally [7] and is often delayed, because of the nonspecific clinical presentation, which can suggest only an inflammatory process [4]. The time from the initial problem to presentation averages approximately 24 months [6]. In an extensive review of reported cases, 54% of patients had metastases at diagnosis with lymphatic spread to the lumboaortic and iliac nodes and metastases to the bones, lungs, and skin [1]. The common sites of early metastases are the lymph nodes, lungs, liver, and bones [2]. Metastatic adenocarcinoma from another site must be ruled out. PET-CT may provide improved diagnostic sensitivity over conventional CT [8]. Diagnosis of this tumor is based on well-established criteria. The diagnostic criteria of Nochomovitz and Orenstein [5] for adenocarcinoma of the rete testis include 1) tumor origin around the hilum, 2) no involvement of the tunica, 3) demonstration of transition from normal to tumor epithelium, and 4) no evidence of teratoma or any other primary tumor elsewhere in the body. Our case fit 3 of these 4 diagnostic criteria: 1), 2), and 4). Most rete testis adenocarcinomas have a papillary pattern [4]. The tumor cells are cuboidal or columnar with acidophilic or amphophilic cytoplasm [9]. A few cases have been examined immunohistochemically and are positive for cytokeratins and negative for beta-hCG, PSA, and alpha-fetoprotein [1]. Positivity for CEA may be seen, and it is helpful in excluding the diagnosis of mesothelioma [1]. In our case, thyroid transcription factor-1 was negative, which is a useful marker for lung cancer [3]. However, reliable markers that can define the origin of the adenocarcinoma as the rete testis have never been indicated.
Radical orchiectomy is assumed to be the primary treatment modality for these patients [10]. Sanchez-Chapado et al. [4] reported that retroperitoneal lymph node dissection has merit whenever the tumor is resectable. In advanced stages, adenocarcinoma of the rete testis is highly resistant to adjuvant radiotherapy and any known chemotherapy. The natural history of this tumor appears to be highly malignant, with 3- and 5-year disease-free survival rates of 49% and 13%, respectively. About 40% of patients die within 1 year of diagnosis. Our patient died only 8 months after diagnosis. The most important prognostic factor is the size of the primary tumor. The stage at diagnosis does not have a significant influence by itself [3].




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download