Abstract
Purpose
The present study was done to define the degree of intravesical prostatic protrusion (IPP) causing bladder outlet obstruction (BOO) in patients with benign prostatic hyperplasia (BPH)/lower urinary tract symptoms.
Materials and Methods
We retrospectively evaluated 239 patients with BPH, analyzing age, IPP, prostate volume, International Prostate Symptom Score (IPSS), and the results from a pressure-flow study. Urethral resistance was quantified by using the BOO index (BOOI), according to the formula BOOI=PdetQmax-2×Qmax (where Pdet is detrusor pressure at the peak flow rate and Qmax is peak flow rate). BOO was defined by a BOOI above 40. Patients with a BOOI below 20 were excluded. Patients were classified into two groups (obstructed and unobstructed groups) by the BOOI. Correlations were determined by logistic regression analysis, and receiver operating characteristic curves were plotted to estimate the optimal cutoff for IPP.
Results
There were significant differences in total prostate volume, postvoiding residual urine (PVR), IPP, and Qmax (p<0.001, p<0.001, p<0.001, and p=0.026, respectively) between the obstructed and unobstructed groups, but there were no significant differences in age (p=0.653), IPSS total score (p=0.624), or quality of life score (p=0.138). IPP had a significant prognosis (p<0.001) but was weakly correlated with prostate volume (p=0.026). The correlation coefficients between IPP and Qmax, PVR, prostate volume, and BOO were 0.551, -0.159, 0.225, and 0.391, respectively. For IPP, the area under the curve was 0.759 (95% confidence interval, 0.657 to 0.861) and the cutoff to indicate BOO was 5.5 mm with 66.7% sensitivity and 80.5% specificity.
Parameters predicting bladder outlet obstruction (BOO) in men with lower urinary tract symptoms (LUTS) include detrusor pressure and maximal urine flow rate in pressure-flow studies [1]. However, pressure-flow studies are invasive because catheterization is required to measure detrusor pressure and are uncomfortable for patients because of pain and voiding difficulty [2].
Recent studies have reported on noninvasive parameters predicting BOO in men with LUTS. An anatomical configuration of the prostate termed the intravesical prostatic protrusion (IPP) can be measured as the vertical distance from the tip of the protruding prostate to the base of the bladder at the base of the prostate gland. The IPP can be measured noninvasively by transrectal ultrasonography (TRUS) and can predict voiding parameters for determining BOO in men who present with LUTS. IPP may also be a useful predictor for clinical progression in men with benign prostatic hyperplasia (BPH) [3-6].
There are no definitive studies concerning the specific degree of IPP that causes BOO. IPP is insufficient for managing patients with BPH/LUTS; physicians know only that IPP is a significant predictor for BOO, because most elderly people with BPH/LUTS have some degree of IPP. Hence, a novel means of gauging IPP is needed. Furthermore, there have been no studies with a large Korean population.
The goal of this study was therefore to determine noninvasive parameters for predicting BOO and defining the degree of IPP causing BOO in a large population of Korean men by analyzing the receiver operating characteristic (ROC) curves for IPP, including the cutoff, and correlating IPP and other clinical variables with the results of a pressure-flow study.
We performed a retrospective analysis of clinical outcomes of consecutive male Korean patients aged 50 to 90 years with LUTS/BPH who underwent transurethral resection of the prostate (TURP) from May 2007 to December 2010. Patients with a known history of prostate or bladder carcinoma, urinary tract infection, renal impairment, bladder calculi, or neurological deficit were excluded.
Survey of the International Prostate Symptom Score (IPSS) and quality of life (QoL) scores was performed for evaluation of baseline parameters. A physical examination included a digital rectal examination to screen for prostatic growth suspicious of malignancy and a neurological examination to exclude any neurological deficits or neurologically related bladder dysfunction.
Preoperative evaluation included urodynamic studies including pressure-flow studies and serum prostate-specific antigen (PSA). Four urodynamic parameters including the postvoiding residual urine (PVR), peak flow rate (Qmax), detrusor pressure at the peak flow rate (PdetQmax), and maximal detrusor pressure during voiding (Pdetmax) were statistical analyzed. Urethral resistance was quantified by using the BOO index (BOOI), according to the formula BOOI=PdetQmax-2×Qmax [7]. The bladder was defined as obstructed when the BOOI was >40. Patients with a BOOI <20 were excluded. Patients were classified into two groups by the BOO: obstructed (BOOI>40) and unobstructed (BOOI≤40).
TRUS was performed to measure IPP, prostate volume, and transitional zone volume. IPP was also measured as the vertical distance from the tip of the protruding prostate to the base of the bladder at the base of the prostate gland (Fig. 1) [6].
Spearman's rank correlation was used to identify univariate associations between factors. ROC curve analysis was performed to evaluate the diagnostic performance of IPP for predicting BOO. A multivariate logistic regression was performed to identify strength and association between BOO and IPP, compensating for factors such as age, IPSS, QoL, prostatic volume, and urodynamic parameters. SPSS ver. 13. 0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. A p-value below 0.05 was considered to statistically significant.
Of the 534 patients, 239 patients were included and 295 patients were excluded because of neurologic deficits, urinary tract carcinoma, or renal impairment. The mean age of the 239 included patients was 69.9±8.0 years (mean±standard deviation). The mean IPSS was 14.2±11.6, the mean Qmax was 9.5±6.8 mL/s, the mean PVR was 35.1±46.6 mL, and the mean degree of IPP was 3.42±4.71 mm. The number of patients in the unobstructed and obstructed groups was 193 and 46, respectively. Significant BOO (index>40) was associated with higher IPP (8.08±6.74 mm, p<0.001) compared with equivocal BOO (1.98±3.11 mm, p<0.001).
The area under the ROC curve (AUC) for IPP was 0.759 (95% confidence interval [CI], 0.657 to 0.861) (Fig. 2). At the best cutoff for IPP of 5.5 mm, the sensitivity was 66.7% and the specificity was 80.5%. The AUCs for total prostate volume, serum PSA, Qmax, and PVR were 0.746, 0.572, 0.384, and 0.696, respectively. All areas were lower than that of the IPP.
BOO was positively correlated with IPP, PVR, and prostate volume (Spearman's rho=0.551, 0.225, and 0.391, respectively; p<0.001 for each parameter) and was negative correlated with Qmax (Spearman's rho=-0.159, p=0.026). The correlations of the various clinical variables with BOO are summarized in Table 1. IPSS and the QoL index had poor predictive significance for BOO (positive predictive value of <60%, p=0.624 and p=0.138, respectively).
Qmax, PVR, prostatic volume, and IPP had significant predictive value for BOO (p=0.026, p<0.001, p<0.001, and p<0.001, respectively). However, Qmax, PVR, and prostatic volume had lower positive predictive values (27% to 53% vs. 79%) than did IPP, and the negative predictive value of Qmax, PVR, and prostatic volume were similar to that of IPP (87% to 91% vs. 89%).
Logistic regression analysis revealed that IPP was a significant independent variable when other covariates including age were considered. The odds ratio of BOO for IPP was 3.99 (95% CI, 1.46 to 10.8) (Table 2).
Symptomatic BPH is one of the most common diseases in elderly men. One of the important pathophysiologies in BPH is BOO, making evaluation of the severity and presence of BOO important. There is no definite or reference standard for BOO, except for pressure-flow study. However, the latter is too expensive (especially in developing countries), is invasive, and is uncomfortable for patients. To avoid these drawbacks, the identification of new accurate methods that could substitute for the gold standard pressure-flow study at lower cost, expanded accessibility, and with relief of patient discomfort has become an important goal.
Other noninvasive clinical variables have no significant correlation with BOO [5,8-10]. The IPSS is a simple tool in the evaluation of benign prostatic enlargement, and a worsening score may warrant intervention. However, its poor correlation with BOO is a major drawback [8,10]. PVR may reflect the severity of BOO, but the presence of bladder dysfunction confounds its value [11].
In our study, IPSS and QoL score were not correlated with BOO (p=0.624 and p=0.138, respectively). This was expected, because symptoms need not be related to obstruction. In addition, the urine flow rate and PVR had lower positive predictive values than did IPP. Clinical data such as IPSS, PVR, and flowmetry correlate mostly to lower urinary tract functional status rather than mechanical obstruction itself [8-10].
IPP aside from pressure-flow studies is a predictor of BOO in BPH/LUTS patients. IPP arises from the enlargement of the median and lateral lobes and causes a ball-valve type of obstruction, which thus disrupts the funneling effect of the bladder neck. Urethral resistance and bladder dyskinetic movement in voiding are consequences. The severity of IPP has been correlated with prostate volume, creating BOO in patients with BPH/LUTS. In the present study, the BOO group displayed a greater IPP than did the equivocal BOO group (p<0.001), and the degree of IPP correlated with BOO (r=0.551). Despite this, we analyzed the specific degree of IPP causing BOO. Analysis of the ROC curve concerning the relationship between IPP and BOO revealed a cutoff of 5.5 mm for IPP (sensitivity of 66.7% and specificity of 80.5%): an IPP exceeding 5.5 mm was significantly associated with BOO.
BPH/LUTS patients display some degree of IPP measured by ultrasonography. But the finding that IPP exists in BPH/LUTS patients cannot completely explain the situation in patients in whom symptoms are intractable to treatment with alpha-blockers or TURP. We think that a specific degree of IPP can be applied in the treatment of BPH/LUTS patients. Further studies are needed to define a cutoff of IPP as a causative criterion of BOO. Keqin et al. [3] and Reis et al. [12] reported that ROC curves of IPP yielded an AUC of 0.858 and 0.758, respectively, which are similar or greater than the presently found value. Keqin et al. [3] reported that the best cutoff was 7.5 mm (sensitivity of 75.5% and specificity of 82.6%), which was greater than our cutoff of 5.5 mm.
The patients in our study were Korean males who presented with a complaint of LUTS. There could be a racial explanation for the difference in cutoff values between the prior and present studies. Also, the study number (n=239) was small; studies with larger numbers are warranted. Yet, the present study corroborates the findings of prior studies concerning the IPP cutoff for BOO.
We analyzed ROC curves concerning the relationship between IPP and BOO and between BOO and other parameters (prostate volume, PSA, Qmax, PVR). IPP had a greater value of AUC than did the other parameters. Interestingly, the IPP displayed a greater AUC value of 0.759 than did prostate volume (0.746). The prevailing view is that IPP correlates with BOO but does not have a significant value compared with prostate volume.
The presently determined AUC value for IPP indicates that the measurement of IPP has greater diagnostic value in BOO than does prostate volume in evaluating prostate sonographic data. BOO is dynamic and is influenced by the physical obstruction of the bladder and prostate, and we think that IPP measurement is needed in patients when prostate volume is not excessive, and that BOO patterns may be useful. Also, IPP measurements besides uroflowmetry, PVR, and PSA likely are influential in diagnosing BOO, in that in the present study IPP produced a larger AUC than did PSA, Qmax, or PVR.
These data support the view that IPP can predict BOO, compared with Qmax, PVR, and prostate volume, for BPH/LUTS patients, and may have diagnostic predictive value similar to that of pressure-flow studies. Also, by predicting BOO and defining a specific IPP cutoff linked with the occurrence of BOO, we suggest that the degree of IPP can be a guideline for further treatment in patients with BPH/LUTS.
Figures and Tables
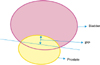 | FIG. 1Schematic (ultrasound) image used in the grading system for intravesical prostatic protrusion (IPP). IPP is measured as the vertical distance from tip of the protrusion to the base of the bladder. |
 | FIG. 2Receiver Operating Characteristic curve for IPP and other parameters. IPP, intravesical prostatic protrusion; PSA, prostate-specific antigen; PV, prostate volume; PVR, postvoiding residual urine; Qmax, peak flow rate. |
TABLE 2
The statistical analysis by a logistic regression model (n=239)
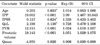
CI, confidence interval; IPP, intravesical prostatic protrusion; IPSS, International Prostate Symptom Score; QoL, quality of life; PVR, postvoid residual urine; Qmax, peak flow rate.
a:Statistically insignificant; the correct classification rate based on a threshold of 0.5 was 90.0%. The Hosmer-Lemeshow goodness-of-fit test showed the model fit well (p=0.95).
References
1. Abrams P. In support of pressure-flow studies for evaluating men with lower urinary tract symptoms. Urology. 1994; 44:153–155.
2. Klingler HC, Madersbacher S, Djavan B, Schatzl G, Marberger M, Schmidbauer CP. Morbidity of the evaluation of the lower urinary tract with transurethral multichannel pressure-flow studies. J Urol. 1998; 159:191–194.
3. Keqin Z, Zhishun X, Jing Z, Haixin W, Dongqing Z, Benkang S. Clinical significance of intravesical prostatic protrusion in patients with benign prostatic enlargement. Urology. 2007; 70:1096–1099.
4. Lee LS, Sim HG, Lim KB, Wang D, Foo KT. Intravesical prostatic protrusion predicts clinical progression of benign prostatic enlargement in patients receiving medical treatment. Int J Urol. 2010; 17:69–74.
5. Chia SJ, Heng CT, Chan SP, Foo KT. Correlation of intravesical prostatic protrusion with bladder outlet obstruction. BJU Int. 2003; 91:371–374.
6. Nose H, Foo KT, Lim KB, Yokoyama T, Ozawa H, Kumon H. Accuracy of two noninvasive methods of diagnosing bladder outlet obstruction using ultrasonography: intravesical prostatic protrusion and velocity-flow video urodynamics. Urology. 2005; 65:493–497.
7. Lim CS, Abrams P. The Abrams-Griffiths nomogram. World J Urol. 1995; 13:34–39.
8. Netto Junior NR, D'Ancona CA, de Lima ML. Correlation between the International Prostatic Symptom Score and a pressure-flow study in the evaluation of symptomatic benign prostatic hyperplasia. J Urol. 1996; 155:200–202.
9. el Din KE, Kiemeney LA, de Wildt MJ, Rosier PF, Debruyne FM, de la Rosette JJ. The correlation between bladder outlet obstruction and lower urinary tract symptoms as measured by the international prostate symptom score. J Urol. 1996; 156:1020–1025.
10. Ezz el Din K, Kiemeney LA, de Wildt MJ, Debruyne FM, de la Rosette JJ. Correlation between uroflowmetry, prostate volume, postvoid residue, and lower urinary tract symptoms as measured by the International Prostate Symptom Score. Urology. 1996; 48:393–397.
11. Mochtar CA, Kiemeney LA, van Riemsdijk MM, Laguna MP, Debruyne FM, de la Rosette JJ. Post-void residual urine volume is not a good predictor of the need for invasive therapy among patients with benign prostatic hyperplasia. J Urol. 2006; 175:213–216.
12. Reis LO, Barreiro GC, Baracat J, Prudente A, D'Ancona CA. Intravesical protrusion of the prostate as a predictive method of bladder outlet obstruction. Int Braz J Urol. 2008; 34:627–633.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download