Abstract
Purpose
The location of acetylcholinesterase-containing nerve fibers suggests a role for acetylcholine in both contractility and secretion in the prostate gland. The colocalization of nitrergic nerves with cholinergic nerves, and the cotransmission of nitric oxide with acetylcholine in cholinergic nerves, has been demonstrated in the prostate glands of various species. Thus, we investigated the effects of acetylcholine on phenylephrine-induced contraction and the correlation between cholinergic transmission and nitric oxide synthase by using isolated prostate strips of rabbits.
Materials and Methods
Isolated prostate strips were contracted with phenylephrine and then treated with cumulative concentrations of acetylcholine. Changes in acetylcholine-induced relaxation after preincubation with NG-nitroarginine methyl ester, 7-nitroindazole, and aminoguanidine were measured. The effects of selective muscarinic receptor antagonists were also evaluated.
Results
In the longitudinal phenylephrine-contracted strip, the cumulative application of acetylcholine (10-9 to 10-4 M) elicited a concentration-dependent relaxation effect. Acetylcholine-induced relaxation was inhibited not only by nitric oxide synthase inhibitors (10 µM L-NAME or 10 µM 7-nitroindazole) but also by 10 µM atropine and some selective muscarinic receptor antagonists (10-6 M 11-([2-[(diethylamino)methyl]-1-piperdinyl]acetyl)-5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepine-6-one and 10-6 M 4-diphenylacetoxy-N-methyl-piperidine). In contrast, relaxation was significantly increased by pretreatment of the strips with 10 mM L-arginine.
The prostate stroma, but not secretory acini, receives rich noradrenergic innervation. Inhibition of smooth muscle contraction is achieved by the use of α1-adrenoceptor antagonists in benign prostatic hyperplasia. The mammalian prostate is innervated by hypogastric and pelvic nerves that play an important role in regulating the growth and function of the gland. Although there has been much interest in the role of noradrenergic innervation and adrenoceptors in prostate function, the role of cholinergic neurons in prostate physiology and pathophysiology is not well understood.
Histochemical staining has revealed that acetylcholinesterase-positive nerves innervate the prostate stroma in addition to the epithelium [1,2]. In vivo activation of muscarinic receptors in the prostate gland causes glandular secretion. However, the effect of muscarinic receptor activation on smooth muscle contraction in stromal tissues remains highly controversial [3].
Nitric oxide synthase (NOS)-positive nerves have recently been detected in human and rat prostates [4], and immunohistochemical studies of dog prostate have revealed that NOS-containing nerves (nitrergic nerves) are distributed in both the stroma and the epithelium [5]. Moreover, nitric oxide (NO) may act as a cotransmitter or modulator in autonomic efferent nerves supplying the prostate stroma [6]. NO-mediated signals have central roles in genitourinary tract relaxation responses and may be directly involved in modulating the smooth muscle tones of the bladder neck and urethra [7,8]. In addition, the prostate, urethra, and bladder neck are also densely supplied by NOS-containing nerves [9]. More recently, immunohistochemical staining revealed that vesicular acetylcholine transporter-positive NOS-containing cholinergic neurons were located predominantly in the detrusor muscle of the bladder body and neck and prostate [9,10]. Dixon et al. [9] have suggested that NO is involved in cholinergic transmission in the prostate stroma.
This research aimed to determine the role of acetylcholine in modulating prostate smooth muscle function and to examine the correlation between cholinergic transmission and NOS by using isolated prostate strips of rabbits.
Experiments were carried out according to guidelines from the Committee for the Protection of Persons and Animals at the Institute of Medical Science at our university in Seoul, Korea.
A total of 30 New Zealand white rabbits (weight, 3 to 4 kg) were used. Rabbits were anaesthetized with an overdose of pentobarbital (60 mg/kg, intraperitoneal) and were then sacrificed by incision of the carotid artery. After cutting of the pubic bone, prostate tissues were harvested and transferred to Petri dishes containing 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffered physiological salt solution (PSS) with 100% O2 saturation.
The surrounding tissue was carefully removed from the prostates, and 1 mm×1 mm×10 mm strips were cut from the center in a longitudinal direction (parallel) to the urethra. Four prostate strips from each animal were obtained; for each experiment, we used just 2 longitudinal strips of the adenomatous tissue surrounding the urethra (Fig. 1). Each strip was suspended in a 30-mL organ bath containing PSS with the following composition: 114 mM NaCl, 26 mM NaHCO3, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM NaH2PO4, and 11 mM D-glucose. During the experiments, the baths were maintained at 37℃ and continuously bubbled with gas containing 95% O2 and 5% CO2, maintaining a pH of 7.3 to 7.4. For the experiments, each prostate strip was connected to a force transducer (52-9545, Harvard Apparatus, London, UK). Analog signals were converted to digital signals, which were recorded on a MacLab 4e recording system (ADInstruments, Bella Vista, Australia).
The strips were maintained at 2 g of resting tension and equilibrated for 2 hours with several changes of PSS at 30-minute intervals.
The following drugs were used in the present study: phenylephrine, acetylcholine, hexamethonium, pirenzepine dihydrochloride, 11-([2-[(diethylamino)methyl]-1-piperdinyl] acetyl)-5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepine-6-one (AF-DX116), 4-diphenylacetoxy-N-methyl-piperidine (4-DAMP), tropicamide, NG-nitroarginine methyl ester hydrochloride, 3-bromo-7-nitroindazole (7-NI), aminoguanidine hydrochloride, and L-arginine. All drugs were purchased from Sigma Chemical Company (St. Louis, MO, USA).
In the resting state, the responses of a strip to various concentrations (10-9 to 10-4 M) of acetylcholine were observed. The strips were first contracted with phenylephrine (10-5 M) for 5 minutes and were then treated with cumulative concentrations of 10-9 to 10-4 M acetylcholine (3 minutes, respectively).
Cholinergic receptor involvement with acetylcholine-induced relaxation was examined by first incubating the strips with 10-5 M hexamethonium (nicotinic receptor antagonist), 10-5 M atropine (a nonselective muscarinic receptor antagonist), and selective muscarinic receptor (M1, M2, M3, M4) antagonists (including 10-6 M pirenzepine, 10-6 M AF-DX116, 10-6 M 4-DAMP, and 10-6 M tropicamide, respectively) for 10 minutes in separate experiments and then recording the acetylcholine-induced relaxation as described.
To investigate whether NOS is involved in the acetylcholine-induced relaxation mechanism, the experimental strips were pretreated with 10 µM NG-nitroarginine methyl ester (L-NAME, nonspecific NOS inhibitor), 10 µM 7-NI (mostly an inhibitor of neuronal NOS [nNOS] but partially an endothelial nitric oxide synthase inhibitor [eNOS]), 10 µM aminoguanidine (irreversible inducible NOS inhibitor), and 10 mM L-arginine (NOS substrate) within 10 minutes before being contracted by 10-5 M phenylephrine. The effects of these agents on acetylcholine-induced relaxation were measured.
Results are presented as mean±standard error of the mean. Maximum contractions elicited by phenylephrine were considered 100% contraction. The relaxation measurement of each prostate strip was expressed as a percentage of the reduction in maximum contraction. IC50 (the half-maximum inhibitory concentration) or EC50 (the half-maximum effective concentration) values were calculated and obtained from regression plots, and each regression plot was constructed with four to five points by using the logistic sigmoidal fitting model (MicroCal Origin, ver. 7.5).
In all experiments, "n" refers to the number of strips. Student's t-tests and repeated-measures analysis of variance were used for the analysis, and p-values of 0.05 were considered statistically significant.
Application of acetylcholine (10-9 to 10-4 M) to strips in the resting state did not evoke any remarkable change (n=4, data not shown). The cumulative applications of acetylcholine to phenylephrine-contracted strips, however, elicited a concentration-dependent relaxation effect (Fig. 2). The acetylcholine IC50 and maximum relaxation values were 45.44±40.66 µM and 39.21%±2.56%, respectively.
Acetylcholine-induced relaxation was inhibited in the strips pretreated with atropine but not in the strips pretreated with hexamethonium (Fig. 3A).
The M2 (10-6 M) and M3 (10-6 M) receptor antagonists almost completely abolished acetylcholine-induced relaxation (Fig. 3B); whereas the M1 (10-6 M) and M4 (10-6 M) receptor antagonists did not influence the acetylcholine effect (data not shown).
Cholinergic nerves, which are present in the prostate of several species, primarily release the transmitter acetylcholine, which activates muscarinic receptors [1]. Some studies, using the acetylcholinesterase reaction for identifying nerve fibers, have shown that cholinergic nerve fibers innervate not only the epithelium but also the stromal tissues [11,12]. Functionally, these nerves have important roles in the regulation of the growth and secretion of the prostate epithelium [6]. An early report indicated that cholinergic nerve stimulation increases prostatic secretion in dogs [13]. Another study using the cholinergic agonist carbachol also demonstrated increased secretion by the rat prostate [3]. Acetylcholine induces strong inwardly rectifying currents in both sympathetic and parasympathetic major pelvic ganglia neurons, thus providing autonomic innervation to the prostate [14]. A predominantly epithelial location for prostate muscarinic receptors indicated a major secretomotor role for acetylcholine. However, many reports have suggested a role for acetylcholine in contractility in various parts of the prostate gland of different species, including humans, but the effect is small relative to that produced by alpha-adrenoceptor agonists [6].
In the rabbit prostate, the capsular muscles are composed of thick and thin muscle bundles, within which the distribution of muscarinic receptors differs. In the thin muscle bundles, acetylcholine depolarizes the membrane of cells and produces phasic and tonic contractions that are blocked by atropine. However, in the thick bundles, acetylcholine neither depolarizes the membrane nor produces any mechanical responses [1]. In the dog prostate, both acetylcholine and carbachol produce a contraction of the capsular strips but not of strips obtained from the internal zone of the gland [12]. In contrast, another study reported that acetylcholine causes contraction of only one preparation of isolated dog prostate; the other preparations that were studied were not sensitive [13]. Furthermore, Smith et al. [15] found that acetylcholine elicited no contractile effect in either capsular or noncapsular strips of dog prostate. Similarly, in human prostatic adenoma, carbachol produced a weaker (<15%) contractile response than that produced by phenylephrine [16], although others have reported that both acetylcholine and carbachol failed to cause contraction of such preparations [17,18]. In contrast to adenoma tissue, the prostatic capsule of patients with benign prostatic hypertrophy contract strongly in response to acetylcholine, and that response is inhibited by atropine [17].
In our study, the relaxation response induced by acetylcholine on phenylephrine-precontracted strips was seen only in the longitudinal strips. Our data are not consistent with those reported previously because the effects of acetylcholine on prostate strips may depend on the species or on the part of the lobe. It is of interest that different densities of muscarinic receptors have been found in various lobes of the rat prostate gland [19] and that differences are also found in the levels of growth factors in the various lobes of the guinea pig prostate [20]. Furthermore, it is thought that because previous experiments were performed in the resting state, the relaxation effects of acetylcholine could not have been observed. To our knowledge, these effects were recorded for the first time, which suggests a role for acetylcholine in inducing relaxation in precontracted prostate strips by sympathomimetics.
In the present study, acetylcholine-induced relaxation was completely inhibited by 10-5 M atropine but not by 10-5 M hexamethonium. These findings indicate that acetylcholine exerts a direct relaxation effect on rabbit prostate smooth muscle by the activation of muscarinic, but not nicotinic, receptors. Protein and mRNA studies have revealed the presence of 5 muscarinic receptor subtypes in human, guinea pig, dog, rat, and rabbit prostates with different species-dependent roles in regulating prostate smooth muscle function [6]. M1, M2, and M3 muscarinic receptors have been implicated in eliciting or facilitating contraction in guinea pig, dog, and rat prostate tissue, respectively [1]. In humans, there is evidence for M1 receptors on the epithelium, M2 receptors on the stroma, and both M1 and M3 receptors in some prostate cancer cell lines [6]. In rabbits, the muscarinic receptor detected in binding studies was shown to have a pKi value of ~7.5 for the M1 muscarinic receptor antagonist [21]. Because no other selective antagonists have been studied in rabbit prostate, we have no indication of the nature of the receptor subtype involved. Our study identified M2- and M3-selective muscarinic receptor antagonists that inhibited acetylcholine-induced relaxation.
The presence of nitrergic nerves, the third type of neuron in the autonomic nervous system, can be demonstrated in the lower urinary tract [7]. Some recent reports have also detected nitrergic nerves in dog, human, rat, rabbit, and pig prostates [5]. Immunohistochemical staining demonstrated that nitrergic nerves were located in both the stromal and the epithelial components of dog prostate and were mainly present in the ventral rat prostate [5]. In humans, the presence of these nerves was indirectly identified by measuring NOS activity, which was revealed to be higher in the peripheral zone than in the transition zone [22]. Compared with pig and rat, NOS activity was greater in the rabbit prostate [23]. Interestingly, we found evidence of nitrergic nerves colocalizing with cholinergic and adrenergic fibers. Hedlund et al. [5] demonstrated the colocalization of NOS in cholinergic nerves supplying the dog prostate. In a subsequent study, he also reported the colocalization of cholinergic nerves with nitrergic nerves in the human prostate [24]. In addition, cotransmission of NO with the neurotransmitters acetylcholine, noradrenaline, and other neuropeptides from the nerves supplying the prostate of various species, including dogs, guinea pigs, and humans, has also been demonstrated [1,9].
The results of the present study show that acetylcholine-induced relaxation was almost completely inhibited by pretreatment with 10-6 M L-NAME, but was significantly increased by 10 mM L-arginine. These responses are thought to be attributable to nitrergic innervations that are readily demonstrated in strips of rabbit prostate. There are three isoforms of NOS: inducible NOS (iNOS), nNOS, and eNOS. Of these, nNOS and eNOS are constitutively expressed in many tissues. Pretreatment with 10 µM aminoguanidine (an irreversible iNOS inhibitor) did not affect acetylcholine-induced relaxation, whereas pretreatment with 10 µM 7-NI (a mostly nNOS but partially eNOS inhibitor) considerably inhibited it. These results indicate that acetylcholine-induced relaxation of prostate strips occurred via the constitutive NOS-mediated release of NO. Some studies on isolated preparations of rabbit, canine, and human prostates have shown that nerve stimulation-induced relaxation was also abolished by NOS inhibitors [4,24]. In another study, after excluding noradrenergic and cholinergic pathways innervating the rabbit prostate, field stimulation relaxed noradrenaline- or methoxamine-precontracted strips, and this relaxation was greatly inhibited by 100 µM L-NAME [23].
In the present study, high concentrations of acetylcholine caused contraction of prostate strips after L-NAME or nitroindazole pretreatment. This result could be explained by the complex interaction of NO and acetylcholine. NO inhibition is likely to affect the action of acetylcholine on prostate smooth muscle strips, directly or indirectly. The evidence showing colocalization of nitrergic nerves with cholinergic and adrenergic fibers, and cotransmission of NO with acetylcholine, noradrenaline, and other neuropeptides from the nerves supplying the prostate, suggests that NO may act directly as a transmitter to cause relaxation of smooth muscle or indirectly as a modulator by facilitating the release of other transmitters that cause relaxation. The exact mechanisms by which NO participates in the regulation of this relaxation remain to be determined.
Acetylcholine relaxes the phenylephrine-induced contraction of isolated rabbit prostate. This relaxation may be mediated by muscarinic receptors, especially M2 and M3. Colocalized NO released by constitutive NOS may also play a key role as a direct transmitter or as a modulator of efferent neurotransmission.
Figures and Tables
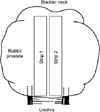 | FIG. 1Schematic representation of the strips from rabbit prostate. Only 2 longitudinal strips (strip 1 and 2) of the adenomatous tissue surrounding the urethra were used in each experiment. |
 | FIG. 2(A) Original trace showing the dose-dependent relaxation responses of a phenylephrine (PE)-contracted strip to acetylcholine (ACh). (B) Relaxation effect of ACh on PE-contracted prostate strips (n=8, Student's t-test). W/O, washout. |
 | FIG. 3(A) Effect of hexamethonium and atropine on acetylcholine-induced relaxation in phenylephrine-contracted strips (n=12, repeated-measures analysis of variance, a:p<0.05). (B) Comparative effects of 11-([2-[(diethylamino)methyl]-1-piperdinyl]acetyl)-5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepine-6-one (10-6 M) and 4-diphenylacetoxy-N-methyl-piperidine (10-6 M), selective M2 and M3 muscarinic receptor antagonists, respectively, on acetylcholine-induced relaxation in phenylephrine-contracted preparations (n=12, repeated-measures analysis of variance, a:p<0.05). ACh, acetylcholine. |
ACKNOWLEDGMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project of the Ministry for Health, Welfare & Family Affairs of the Republic of Korea (A085138).
References
1. Pennefather JN, Lau WA, Mitchelson F, Ventura S. The autonomic and sensory innervation of the smooth muscle of the prostate gland: a review of pharmacological and histological studies. J Auton Pharmacol. 2000. 20:193–206.
2. McVary KT, McKenna KE, Lee C. Prostate innervation. Prostate Suppl. 1998. 8:2–13.
3. Wang JM, McKenna KE, Lee C. Determination of prostatic secretion in rats: effect of neurotransmitters and testosterone. Prostate. 1991. 18:289–301.
4. Takeda M, Tang R, Shapiro E, Burnett AL, Lepor H. Effects of nitric oxide on human and canine prostates. Urology. 1995. 45:440–446.
5. Hedlund P, Larsson B, Alm P, Andersson KE. Nitric oxide synthase-containing nerves and ganglia in the dog prostate: a comparison with other transmitters. Histochem J. 1996. 28:635–642.
6. Ventura S, Pennefather J, Mitchelson F. Cholinergic innervation and function in the prostate gland. Pharmacol Ther. 2002. 94:93–112.
7. Andersson KE, Persson K. Nitric oxide synthase and nitric oxide-mediated effects in lower urinary tract smooth muscles. World J Urol. 1994. 12:274–280.
8. Mumtaz FH, Khan MA, Thompson CS, Morgan RJ, Mikhailidis DP. Nitric oxide in the lower urinary tract: physiological and pathological implications. BJU Int. 2000. 85:567–578.
9. Dixon JS, Jen PY, Gosling JA. The distribution of vesicular acetylcholine transporter in the human male genitourinary organs and its co-localization with neuropeptide Y and nitric oxide synthase. Neurourol Urodyn. 2000. 19:185–194.
10. Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004. 84:935–986.
11. Seki N, Nishiye E, Itoh T, Suzuki H, Kuriyama H. Electrical and mechanical properties of the capsular smooth muscles of the rabbit prostate in relation to the actions of the alpha 1-adrenoceptor blocker, YM-12617. Br J Pharmacol. 1988. 93:702–714.
12. Fernandez JL, Rivera L, Lopez PG, Recio P, Vela-Navarrete R, García-Sacristán A. Characterization of the muscarinic receptor mediating contraction of the dog prostate. J Auton Pharmacol. 1998. 18:205–211.
13. Arver S, Sjostrand NO. Functions of adrenergic and cholinergic nerves in canine effectors of seminal emission. Acta Physiol Scand. 1982. 115:67–77.
14. Park JH, Park KS, Cha SK, Lee KI, Kim MJ, Park JY, et al. Characterization of acetylcholine-induced currents in male rat pelvic ganglion neurons. Korean J Physiol Pharmacol. 2004. 8:219–225.
15. Smith ER, Miller TB, Wilson MM, Appel MC. Effects of vasoactive intestinal peptide on canine prostatic contraction and secretion. Am J Physiol. 1984. 247(4 Pt 2):R701–R708.
16. Lepor H, Gup DI, Baumann M, Shapiro E. Laboratory assessment of terazosin and alpha-1 blockade in prostatic hyperplasia. Urology. 1988. 32:6 Suppl. 21–26.
17. Caine M, Raz S, Zeigler M. Adrenergic and cholinergic receptors in the human prostate, prostatic capsule and bladder neck. Br J Urol. 1975. 47:193–202.
18. Hedlund H, Andersson KE, Larsson B. Alpha-adrenoceptors and muscarinic receptors in the isolated human prostate. J Urol. 1985. 134:1291–1298.
19. Pontari MA, Luthin GR, Braverman AS, Ruggieri MR. Characterization of muscarinic cholinergic receptor subtypes in rat prostate. J Recept Signal Transduct Res. 1998. 18:151–166.
20. Shikata H, Utsumi N, Hiramatsu M, Minami N, Nemoto N, Shikata T. Immunohistochemical localization of nerve growth factor and epidermal growth factor in guinea pig prostate gland. Histochemistry. 1984. 80:411–413.
21. Lepor H, Kuhar MJ. Characterization of muscarinic cholinergic receptor binding in the vas deferens, bladder, prostate and penis of the rabbit. J Urol. 1984. 132:392–396.
22. Burnett AL, Maguire MP, Chamness SL, Ricker DD, Takeda M, Lepor H, et al. Characterization and localization of nitric oxide synthase in the human prostate. Urology. 1995. 45:435–439.
23. Najbar-Kaszkiel AT, Di Iulio JL, Li CG, Rand MJ. Characterisation of excitatory and inhibitory transmitter systems in prostate glands of rats, guinea pigs, rabbits and pigs. Eur J Pharmacol. 1997. 337:251–258.
24. Hedlund P, Ekstrom P, Larsson B, Alm P, Andersson KE. Heme oxygenase and NO-synthase in the human prostate: relation to adrenergic, cholinergic and peptide-containing nerves. J Auton Nerv Syst. 1997. 63:115–126.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download