INTRODUCTION
Among all the procedures used for a permanent urinary diversion, cutaneous ureterostomy (CU) is considered the simplest and safest method. However, after cystectomy for bladder cancer, an ileal conduit is considered the standard form of urinary diversion because CU is associated with a significant risk of stomal stenosis [1]. Nevertheless, if CU can be successfully achieved without a stent catheter, late complications are also reduced [2], and the procedure appears to be as good as the ileal conduit. Various attempts have been made to decrease the frequency of stomal stenosis [2-7]. A high catheter-free rate of 89.8% in 59 renal units (RUs) was reported by the introduction of a new surgical stabilization step for the abdominal wall tunnel in CU [8]. However, there are no diagnostic criteria for evaluating stomal stenosis in CU. The diagnosis is usually established by a combination of clinical symptoms, ultrasonography, excretory urography, and level of serum creatinine (sCr). Therefore, it is possible that a stent catheter could be inserted unnecessarily in an unobstructed stoma. Thus, a specific and non-invasive diagnosis test is needed.
Diuretic renography has become an established non-invasive procedure for determining the severity of obstruction, if any, in patients with upper urinary tract dilatation [9]. Previously, the efficiency of 99mTc-mercaptoacetyltriglycine (MAG3) diuretic renography was reported for evaluating stomal stenosis in tubeless CU [10]. However, that study consisted of 15 patients who had undergone CU, and the hydronephrosis was evaluated with excretory urography at 3 and 6 months after surgery. Therefore, in the present study, we tried to reevaluate the stomal obstruction in 29 patients with an average follow-up period of 41.9 months. We demonstrated the diagnostic criteria for stomal obstruction of CU by use of MAG3 diuretic renography.
MATERIALS AND METHODS
A retrospective review was conducted of medical charts and follow-up data for 32 patients who had undergone CU between October 2005 and July 2011 at Kohka Public Hospital. Of these patients, 29 patients (56 RUs) who were established with tubeless CU 3 months after surgery and with at least 12 months of follow-up were enrolled in this study. The ethics committee of Kohka Public Hospital approved this study and written informed consent was obtained from all patients. The underlying disease was bladder cancer in 27 patients and bladder cancer after retroperitoneoscopy-assisted laparoscopic nephroureterectomy with cuff for unilateral renal pelvic cancer in 2 patients. There were 25 men and 4 women with an average age of 70.4±7.5 years (range, 54 to 87 years). The patients' mean body mass index (BMI) was 22.2±2.8 kg/m2 (16.7 to 27.8 kg/m2). Pathological stages among the 29 patients were as follows: Ois (3) 10.3%; I (7) 24.1%; II (14) 48.3%; III (4) 13.8%; and IV (1) 3.4%.
A CU was constructed after complete cystectomy with or without urethrectomy. In 27 patients, both ureters were used to construct the CU with a unilateral stomal creation (on the right side in 21 and on the left side in 6). In the other 2 patients, one ureter was used to construct the CU (on the right side in one and the left side in the other), because these 2 patients had undergone retroperitoneoscopy-assisted laparoscopic nephroureterectomy with cuff for left or right renal pelvic cancer. The unilateral stoma was successfully created in all 29 patients (on the right side in 22 and on the left side in 7). The surgical procedure described by Straffon et al. [11] was used for these patients while creating the course for the ureters. The ureters were brought through in a completely extraperitoneal manner in all patients. The stoma was created in all patients by the Toyoda method [4]. After surgery, a 6-Fr single-J stent was placed in the renal pelvis through the stoma in all patients. In all cases, the single-J stents were exchanged every 4 weeks and were removed 3 months after surgery, because the stomal conditions were unstable and obstructive in the early phase after surgery [10,12]. Stomal conditions were evaluated with excretory urography at 3 and 6 months after the surgery and with MAG3 diuretic renography at 3 months after the surgery. After the removal of the stent catheter at 3 months after the surgery, excretory urography and MAG3 diuretic renography were performed from 2 to 4 days. Further evaluation or follow-up was determined on a case by case basis by the treating physician.
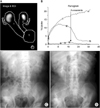
The protocol for MAG3 diuretic renography for CU was described previously [10]. In brief, 60 minutes before scanning, patients were infused with 500 mL normal saline solution intravenously for hydration. MAG3 at a dose of 300 MBq or 8.1 mCi was applied. Regions of interest were drawn that completely encircled and snugly fit the kidney, the renal pelvis, and the ureter (Fig. 1A). When pooling of the tracer was identified near the stoma to permit adequate washout assessment, furosemide (0.5 mg/kg) was injected intravenously. The data analyses were performed with half-times to tracer clearance (T1/2) following furosemide administration and differential renal function (DRF) [13]. The four-grade system was used to evaluate the hydronephrosis in excretory urography [14]. Our definition of the tubeless condition in CU was as follows: 1) the catheter stent is not placed in the renal pelvis through the stoma, 2) the grade of hydronephrosis is less than 3, and 3) the kidney is functioning. The indications for catheter insertion after the establishment of tubeless CU were as follows: 1) difficulty in curing acute pyelonephritis by drug treatments, 2) flank pain due to hydronephrosis, or 3) increase of the grade of hydronephrosis. Student's t-test and the Fisher exact probability test were used for statistical analysis. Significance was defined as p<0.05.
RESULTS
The follow-up period was 12 to 82 months (average, 41.9±23.4 months). When the study ended in July 2012, a total of 21 patients were alive without disease, 4 patients had died of their disease, 1 patient was alive with another type of cancer, and 3 patients had died of other diseases.
Before the surgery, hydronephrosis of grades 1 and 2, respectively, was present in two RUs (3.6%) owing to the ureteral obstruction caused by bladder cancer. After the removal of the single-J stents at 3 months after the surgery, in all 56 RUs that achieved a tubeless condition, 23 (41.1%) had no hydronephrosis. Hydronephrosis of grades 1, 2, and 3 without the need for intervention was present in 13 (23.2%), 18 (32.1%), and 2 RUs (3.6%), respectively. A post-furosemide renogram curve did not decline in one RU (Fig. 1B). Therefore, this RU was excluded in the calculation of T1/2. The T1/2 means were 6.90±6.30, 5.25±4.29, and 8.75±7.63 minutes in the total, ipsilateral, and contralateral kidneys, respectively, in side relationships between the ureter and the stoma (Fig. 2). There were significant differences between the ipsilateral and contralateral kidneys in the T1/2 means (p=0.038). The mean time of furosemide injection after tracer injection was 15.9±4.1 minutes (range, 9 to 25 minutes).
Six months after surgery, of 52 RUs (92.9%) that achieved a tubeless condition, 48 (85.7%) had no hydronephrosis. Hydronephrosis of grades 1 and 2 without the need for intervention was present in one (1.8%) and three RUs (5.4%), respectively. The insertion of the stent catheter was performed in four RUs of three patients. A stent catheter was inserted in two RUs (T1/2: 21.04 minutes and no decline) of two patients owing to both acute pyelonephritis and the persistence of grade 2 hydronephrosis 5 and 4 months after the surgery, respectively. A stent catheter was inserted in two RUs (T1/2: 16.00 and 21.72 minutes) of one patient at 4 months after the surgery. Because this patient experienced repeated pyelonephritis, grades 1 and 2 hydronephrosis persisted, respectively, and his renal function gradually worsened. At the end of the follow-up, of 51 RUs (91.1%) that achieved a tubeless condition, 49 (87.5%) had no hydronephrosis. Two RUs (T1/2: 18.90 and 31.70 minutes) became atrophic, which revealed grades 1 and 2 hydronephrosis, respectively, 6 months after the surgery. A stent catheter was inserted in one RU (T1/2: 20.38 minutes) owing to persistent grade 2 hydronephrosis 14 months after surgery. As shown in Fig. 2, 48 RUs (85.7%) had T1/2 values less than 15 minutes, and they all had no hydronephrosis. On the other hand, 5 RUs (8.9%) had T1/2 values of more than 20 minutes, and these 5 RUs required the insertion of a stent catheter or became atrophic. Three RUs (5.4%) had a T1/2 between 15 and 20 minutes. Each of the 3 RUs had no hydronephrosis, required the insertion of a stent catheter, or became atrophic, respectively.
These results of MAG3 diuretic renography permitted the establishment of criteria for the diagnosis of stomal obstruction of tubeless CU. By use of these criteria, the upper limit of T1/2 for unobstructed systems is 15 minutes, and the lower limit of T1/2 for obstructed systems is 20 minutes. T1/2 values between 15 and 20 minutes indicate equivocal studies. In addition, we recommended the insertion of a stent catheter in RUs that show equivocal T1/2 values and have hydronephrosis at 6 months after surgery. This is because the RU that had grade 1 hydronephrosis 6 months after surgery and a T1/2 of 18.90 minutes became atrophic.
Twenty-seven patients in whom both ureters were used to construct the CU with unilateral stomal creation (21 on the right side and 6 on the left side) were used in the analysis of DRF. The mean DRF values were 52.4%±9.5% (range, 22.0% to 78.0%) and 45.4%±8.3% (range, 22.0% to 78.0%) in ipsilateral and contralateral kidneys, respectively, in side relationships between the ureter and the stoma. In 17 of 27 patients (63.0%), each kidney contributed 45% to 55% of total renal function. There were significant differences between ipsilateral and contralateral kidneys in the mean DRF (p=0.007). All four patients in whom a stent catheter was inserted in one RU or who experienced atrophic change in one RU had abnormal DRF. Six of 22 patients (27.3%) who had two unobstructed RUs also showed abnormal DRF. There were significant statistical differences between the two groups (p=0.014). The DRF revealed abnormal results more often in patients with an obstructed RU than in patients with two unobstructed RUs.
Only one RU did not respond to furosemide in MAG3 diuretic renography (Fig. 1B), and the contralateral RU revealed an unobstructed system (T1/2: 3.40 minutes). However, excretory urography demonstrated bilateral grade 2 hydronephrosis at 3 months after surgery (Fig. 1C). At 4 months after the surgery, this patient experienced left pyelonephritis and an increase in sCr levels from 0.98 to 1.53 mg/dL; thus, the stent catheter was indwelled in the left kidney. Six months after the surgery, the right hydronephrosis improved (Fig. 1D). These results also suggest that MAG3 diuretic renography is more useful than excretory urography for evaluating the stomal obstruction of tubeless CU.
DISCUSSION
After the early phase of surgery, the stoma of a CU is unstable and edematous, resulting in hydronephrosis that is shown by excretory urography. In this study, the excretory urography revealed that 33 of 56 RUs (58.9%) had hydronephrosis after the removal of the stent catheters at 3 months after the surgery. Therefore, it is possible that unnecessary catheterization was performed owing to hydronephrosis after the construction of a CU in patients with acute pyelonephritis. Six months after the surgery, 48 of 56 RUs (85.7%) had no hydronephrosis, which suggests that most of the stomas of the tubeless CU were not obstructed in this study. It is important that reliable diagnostic methods are established for evaluating stomal obstruction of tubeless CU. By performing MAG3 diuretic renography, we were able to develop diagnostic criteria for stomal obstruction of a tubeless CU. As shown in Fig. 2, RUs with a T1/2 of less than 15 minutes clearly showed no hydronephrosis with an average follow-up period of 41.9 months. On the other hand, all RUs with a T1/2 of more than 20 minutes required the insertion of a stent catheter or became atrophic. These results establish that our criteria for diagnosing stomal obstruction of tubeless CU are reliable and are also very useful for the management of CU. It is assumed that an unnecessary insertion of a stent catheter in tubeless CU can be avoided by using our diagnostic criteria for stomal obstruction.
Normally, each kidney contributes 45% to 55% to total renal function [13]. In this study, only 63.0% of the patients showed normal DRF, although most RUs showed unobstructed systems in diuretic renography. In addition, the DRF was significantly normal in more patients with two unobstructed RUs than in patients with one obstructed RU. However, 6 of 22 patients (27.3%) who had two unobstructed RUs also showed abnormal DRF. This proportion was relatively high. Therefore, we conclude that the use of DRF in the evaluation of the stomal obstruction of a tubeless CU with unilateral stomal creation should be avoided until more encouraging results are obtained. There were significant statistical differences between the ipsilateral and contralateral kidneys in both the T1/2 and the DRF. These results suggest that the ureter of a contralateral kidney in a side relationship between the ureter and the stoma might suffer from some stress due to the anomalous course of the ureter.
Because CU is the simplest and safest among all methods of permanent urinary diversions, the frequency of CU performed in elderly patients will increase in an aging society. It is also estimated that the frequency of elderly patients with poor renal function will increase. The shape of the third phase of the renogram curve may be difficult to interpret in patients with poor renal function. Furthermore, impaired renal function may lead to an intermediate or equivocal response to diuretics or a diuretic renogram that is difficult to interpret [15], thus complicating the diagnosis of a functionally significant obstruction. On the other hand, it is very difficult to manage CU with a tubeless condition in patients with poor renal function. It is quite probable that the renal function was worsening, and repeated pyelonephritis occurred in elderly patients with poor renal function. Therefore, CU would result in requiring sustained catheterization with regular catheter exchange before evaluation for the possibility of a tubeless CU in these patients.
CONCLUSIONS
We demonstrated the usefulness of MAG3 diuretic renography for evaluating stomal obstruction in tubeless CU. Our criteria for diagnosing stomal obstruction of tubeless CU by use of MAG3 diuretic renography are as follows: the upper limit of T1/2 for unobstructed systems is 15 minutes, and the lower limit of T1/2 for obstructed systems is 20 minutes. T1/2 values between 15 and 20 minutes indicate equivocal values. In addition, we recommend the insertion of a stent catheter in RUs that both show equivocal T1/2 values and have hydronephrosis at 6 months after surgery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download