Abstract
Purpose
We assessed the predictive factors for renal insufficiency in patients followed for more than 5 years after radical nephrectomy.
Materials and Methods
Age, gender, history of diabetes, history of hypertension, body mass index, preoperative estimated glomerular filtration rate (eGFR), serum uric acid, urine albumin, normal renal parenchymal volume, tumor size, and ratio of normal parenchymal volume of the removed kidney to that of the remaining kidney were evaluated retrospectively in 89 patients who underwent radical nephrectomy from January 2001 to December 2005. Patients were included whose renal parenchymal volume was measurable by use of perioperative imaging (computed tomography or magnetic resonance imaging), whose preoperative eGFR was greater than 60 mL/min/1.73 m2, and who were followed for more than 5 years. To measure renal parenchymal volume from imaging, we integrated the extent of the normal renal parenchyma from axial slides of images.
Results
In univariate and multivariate binary regression analysis, the parenchymal volume of the remnant kidney (p=0.001), a history of diabetes (p=0.035), and preoperative eGFR (p=0.011) were independent factors for renal insufficiency. By use of a receiver operating characteristic curve, a volume of 170 mL was determined to be an appropriate cutoff value, with sensitivity of 58.7% and specificity of 74.4% for the parenchymal volume of the remnant kidney for predicting eGFR less than 60 mL/min/1.73 m2 (area under the curve, 0.678). The parenchymal volume of the remnant kidney was also an independent factor for the downgrading of the chronic kidney disease category in the multivariate linear regression analysis (p=0.021).
Conclusions
Preoperative eGFR, a history of diabetes, and the radiologic volume of the remaining kidney parenchyma could be useful factors for predicting postoperative renal function. Patients with parenchymal volumes of less than 170 mL have a higher risk of postoperative renal insufficiency, which should be considered carefully when choosing a treatment modality.
Many studies have reported that patients with renal cell carcinoma (RCC) may have underlying risk factors for chronic kidney disease (CKD) [1-4]. Barlow et al. [1] suggested that patients undergoing renal surgery have a high rate of new-onset renal insufficiency. Understanding the possibility of renal function change in RCC patients before making any treatment decisions is important because it has been proven that CKD leads to anemia, renal osteodystrophy, uremic malnutrition, hyperlipidemia, and cardiovascular disease [5].
For many years, radical nephrectomy has been considered the treatment of choice for localized renal cortical tumors. However, over the past decade, advances in imaging have allowed for the detection of small, asymptomatic renal tumors, which has altered the management of renal tumors less than 4 cm in size to focus on preventing postoperative renal insufficiency.
Many studies have reported the oncological efficacy and functional advantages of nephron-sparing surgery [1,6-8], and it has been a preferred treatment for small renal tumors. However, in cases with large, endophytic renal tumors, radical nephrectomy is still the gold standard unless the patient has only one kidney or bilateral renal tumors. However, cumulative experience with partial nephrectomy has revealed challenges in such cases. Many recent studies have found that nephron-saving surgery applied to tumors greater than 4 cm provides equivalent oncologic efficacy and superior renal functional outcomes compared with radical nephrectomy [9,10]. This has allowed for flexibility in choosing the appropriate surgical procedure for patients, especially those who have a higher probability of postoperative renal insufficiency. Therefore, it is important to consider predictive preoperative factors for renal insufficiency before renal surgery and to predict postoperative renal function in patients with RCC.
Recently, some studies have reported that the parenchymal volume, which is measured by use of computed tomography (CT), strongly correlates with differential renal function on nuclear renal scanning for normal or chronically obstructed kidneys [11]. A significant correlation was identified between preoperative kidney volume and the glomerular filtration rate (GFR) as well as between postoperative kidney volume and GFR for the unaffected kidney after unilateral nephrectomy [12].
We investigated the predictive preoperative factors for renal insufficiency following radical nephrectomy by focusing on the association between the measured parenchymal volume of the unaffected and the affected kidneys before radical nephrectomy and the postoperative estimated GFR (eGFR) in RCC patients.
Two hundred thirty-six patients who underwent radical nephrectomy for RCC between January 2001 and December 2005 at a single center were included in our study. Patient characteristics, including age, gender, and body mass index (BMI), were measured at hospital admission. Contrast-enhanced CT or magnetic resonance imaging (MRI) was performed and serum creatinine levels were measured before and 5 years after surgery. The GFR was estimated by using the Modification of Diet in Renal Disease (MDRD) formula:
MDRD eGFR=186×(serum creatinine)-1.154 × age-0.203 × (0.742 if female)
All individuals with an MDRD eGFR of less than 60 mL/min/1.73 m2 at 5 years after radical nephrectomy were classified as having renal insufficiency.
We excluded those patients whose renal parenchymal volumes were not measurable by use of a perioperative imaging modality (CT or MRI) or whose eGFR was less than 60 mL/min/1.73 m2 before surgery. After patients with the aforementioned conditions were excluded, 89 patients were included in our study.
We categorized patients into 2 groups on the basis of the eGFR 5 years after radical nephrectomy: eGFR <60 mL/min/1.73 m2 (group A) and eGFR ≥60 mL/min/1.73 m2 (group B).
Kidney volume before radical nephrectomy was measured by using CT or MRI (CT: GE 64 channel VCT, GE Healthcare, Waukesha, WI, USA; MRI: Archieva 3.0T TX, Philips, Best, The Netherlands) by using the standard clinical abdominopelvic imaging protocol for CT and the kidney imaging protocol for MRI in our institution. Venous scans of entire abdomens for CT scan were performed with a 70-s delay after starting the 2-mL/kg intravenous injection of iodinated contrast agent through an antecubital vein. In MRI, T1- and T2-weighted scans of the kidney were performed with a 180-s delay after starting the 0.1-mL/kg intravenous injection of gadolinium contrast agent through an antecubital vein.
All axial images were checked by one urologist who was blinded to patient characteristics in order to distinguish normal-functioning tissue excluding tumor tissue or nonenhanced areas from the axial side of the delayed CT or MRI images with a slice thickness of 5 mm (Fig. 1). The process of distinguishing the tissue depended only on the gross appearance of the images.
The two-dimensional extent of normal-functioning tissue from each image was measured by hand, and the kidney volume was calculated by integrating that extent of normal-functioning tissue.
Preoperative factors such as age, gender, history of diabetes, history of hypertension, body mass index, preoperative eGFR, serum uric acid, urine albumin, normal renal parenchymal volume, tumor size, and the ratio of the normal parenchymal volume of the removed kidney to that of the remaining kidney were compared between groups A and B. For the comparison, the Mann-Whitney U test was used for continuous variables and the chi-square test was used for categorical variables. Predictive factors for renal insufficiency 5 years after radical nephrectomy were analyzed by using univariate and multivariate logistic binary regression.
Because the eGFR 5 years after radical nephrectomy can be influenced by the preoperative eGFR, we reanalyzed the preoperative predictive factors for the downgrading of CKD stage on the basis of the eGFR (Table 1), which may be a more objective measure of kidney function change. For that purpose, 89 patients were categorized into 2 groups: downgrade in CKD stage (group C) and no change in CKD stage (group D).
A receiver operating characteristic (ROC) curve was plotted to determine the cutoff value of the parameters found to be significant for predicting an eGFR greater than 60 mL/min/1.73 m2 at 5 years after radical nephrectomy.
All calculated p-values were two-sided, and values less than 0.05 were considered statistically significant. All data analyses were performed with IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA).
The clinical characteristics of the 89 patients included in the present study are shown in Table 2. On the basis of the eGFR 5 years after radical nephrectomy, 43 patients with an eGFR of less than 60 mL/min/1.73 m2 (group A) and 46 patients with an eGFR of 60 mL/min/1.73 m2 or higher (group B) were compared. Of these patients, 61 were men and 28 were women. The median age of the patients was 61 years (range, 53 to 68 years) in group A and 48 years (range, 42 to 57 years) in group B. The median parenchymal volume of the remnant kidney was 156.28 mL in group A and 181.82 mL in group B.
Median age (61.0 years vs. 48.0 years, p<0.001), preoperative eGFR (73.97 vs. 83.95, p<0.001), serum uric acid level (5.5 vs. 4.3, p=0.011), and parenchymal volume of the remnant kidney (156.28 vs. 181.92, p=0.003) were significantly different between groups A and B.
Table 3 shows the results of the univariate and multivariate binary regression analysis to identify the predictive factors for renal sufficiency (an eGFR less than 60 mL/min/1.73 m2) 5 years after radical nephrectomy. With control for BMI, urine albumin, and serum uric acid, the parenchymal volume of the remnant kidney (odds ratio [OR], 9.185; p=0.001), a history of diabetes (OR, 20.129; p=0.035), and preoperative eGFR (OR, 0.929; p=0.011) showed an association with renal insufficiency.
Because eGFR 5 years after radical nephrectomy was found to correlate with the parenchymal volume of the remnant kidney, an ROC curve was plotted by using data from group A and group B (Fig. 2). The area under the curve was 0.678 (95% CI, 1.677 to 10.189). The optimal cutoff value of the parenchymal volume of the remnant kidney was 170 mL with a sensitivity of 58.7% and a specificity of 74.4% for predicting an eGFR of less than 60 mL/min/1.73 m2.
The clinical characteristic of the 89 patients categorized by change in CKD stage are shown in Table 4. Sixty-one patients showed a downgrade in CKD stage (group C) and 28 patients showed no change in CKD stage (group D). The median parenchymal volume of the remnant kidney was 159.33 mL for group C and 185.91 mL for group D. No parameters were significantly different between groups C and D.
Table 5 provides the results of the univariate and multivariate binary regression analysis to identify the predictive factors for the downgrading of CKD category 5 years after radical nephrectomy. Only the parenchymal volume of the remnant kidney was statistically significant for the downgrading of CKD category in the multivariate linear regression analysis (OR, 3.164; p=0.021). No statistically significant association was identified for preoperative eGFR (p=0.063).
It has been noted that a decrease in the eGFR is associated with an increased risk of cardiovascular events and many other complications [5,14]. Many studies have found that patients with RCC have a high rate of renal insufficiency and that renal surgery, the treatment of choice for renal tumors, can negatively affect postoperative renal function [1-4]. Nephron-sparing surgery is an option that can reduce the chance of postoperative renal insufficiency if it is technically feasible, especially in patients who have more risk factors for postoperative renal insufficiency.
Recently, as experience with partial nephrectomy has accumulated, nephron-saving surgery has been performed even for large (>4 cm), endophytic tumors. Many studies have reported satisfactory results [9,10], which suggests that it is possible to perform nephron-saving surgery for more patients. However, nephron-saving surgery is still technically more difficult than radical nephrectomy for large (>4 cm), endophytic renal tumors and can result in more postoperative complications, a longer operation time, and greater estimated blood loss.
Determining the preoperative risk factors and predicting renal insufficiency after radical nephrectomy are important for patients with large, endophytic tumors and could allow nephron-saving surgery to be performed in patients with a higher risk of renal insufficiency, even those at a higher risk of complications after partial nephrectomy. Several studies have been conducted to identify the predictors of CKD or renal insufficiency after radical nephrectomy. Older age, hypertension, smoking, low preoperative renal function, and diabetes have all been identified as potential risk factors for renal dysfunction [2].
Recently, functional kidney volume has been identified as an additional predictor for postoperative renal function deterioration. Studies have reported a correlation between the percentage functional volume preservation after renal surgery and the percentage decrease in the GFR [15,16]. Simmons et al. [17] reported that preoperative nephron volume and percentage functional volume preservation are the primary determinants of long-term functional outcomes. Regarding donor nephrectomy, Jeon et al. [18] reported that preoperative kidney volume measurement could be used to predict delayed kidney function recovery. These findings suggest that RCC patients with insufficient normal renal parenchymal volume could have a higher rate of renal insufficiency after radical nephrectomy and that surgeons should consider partial nephrectomy in these cases.
In order to use the functional volume of either the affected or the normal kidney as a predictor of renal insufficiency after the surgery, proper measurement of the parenchymal volume of the kidney is important. Because immediate adaptation, such as hypertrophy and hyperfiltration, occurs in the normal kidney after unilateral nephrectomy, several methods have been used by different groups to identify hypertrophy by use of imaging technologies in donors and patients with renal diseases. For example, Gomez-Anson et al. [19] used image-directed color Doppler ultrasonography and identified a mean hypertrophy of approximately 20%. However, the ellipsoid method is less accurate and is affected both by interobserver and intraobserver variability [20,21]. Mullerad et al. [22] evaluated renal hypertrophy in 42 patients (mean age of 61.5 years) after they underwent unilateral nephrectomy by using a dimercaptosuccinic acid renal scan. Recently, CT and MRI have been used to accurately assess functional kidney volume, because these techniques can exclude nonfunctional tissues such as the great renal vessels, perirenal fat, central sinus fat, and renal mass, which allowed for accurate functional kidney parenchymal assessment in our study.
Recent studies have shown that tumor size is a significant risk factor for new-onset renal insufficiency in patients treated with radical nephrectomy [20]. In a study by Ohno et al. [23], the incidence of renal function deterioration (greater than 30% GFR decrease) was higher in patients with tumors sized 7 cm or less than in those with tumors greater than 7 cm (74.7% vs 32.8%, p<0.001). In our study, however, no significant association was identified for tumor size in the univariate analysis (OR, 0.913; p=0.278).
We focused on the influence of the absolute and relative renal parenchymal volume of the remaining kidney on postoperative eGFR. A ratio (parenchymal volume of remnant kidney/total parenchymal volume) of more than 0.5 and less than 0.5 had no significant difference in the univariate analysis (OR, 1.231; p=0.629). Nonetheless, when the absolute renal parenchymal volume of the remaining kidney is larger, especially when the measured volume was greater than 170 mL, the chance of postoperative renal insufficiency (OR, 9.185; p=0.001) or CKD downgrading (OR, 3.164; p=0.021) was significantly lower. We suggest that patients with an unaffected kidney that is already hypertrophied and hyperfiltrated have a lower chance of postoperative renal insufficiency. The recent study by Funahashi et al. [24] supports this conclusion. They reported that a group with an increase in kidney volume of less than 15% at 1 week after surgery as compared to the presurgical volume showed a 4.1-fold percentage increase in their risk of experiencing a 10% reduction in their postsurgical GFR at 6 months compared to the group who experienced an increase in kidney volume of greater than or equal to 15%.
When undergoing renal surgery, patients should be informed of the renal functional outcome in addition to the oncological outcome so that they may make an informed preoperative treatment decision. We believe that our predictive model for new-onset renal insufficiency is useful for preoperative patient counseling.
However, our study had several limitations. First, the patients analyzed in this study represent a retrospective, single-institution experience. Furthermore, the lack of randomization introduces a significant selection bias in the observed functional outcomes of patients treated with partial nephrectomy versus radical nephrectomy.
Second, in order to differentiate the nonenhanced area of the kidney from the CT and MRI scans, we depended on one urologist's opinion about the gross appearance of the images. This could have resulted in bias in our results.
Third, there is no standard method currently available for measuring kidney volume by CT or MRI scan. Consequently, the method of measurement could introduce variability.
Last, prospective data are needed to validate our predictive model for renal insufficiency and to analyze postoperative renal function longitudinally.
The results of this study suggest that preoperative eGFR, history of diabetes, and the radiologic volume of the remaining kidney parenchyma could be useful factors to predict postoperative renal function. Patients with a remaining kidney parenchymal volume of less than 170 mL should be considered candidates for nephron-sparing surgery if technically feasible, because this group has a higher chance of downgrading of CKD category 5 years after radical nephrectomy.
Figures and Tables
FIG. 1
Kidney volume was assessed using personal computer-based software (PACS; Centricity Enterprise Web ver. 3.0). The volume was calculated by integrating the extent of normal functioning tissue, excluding tumours or nonenhanced areas, from the axial side of the computed tomography (CT) or magnetic resonance imaging (MRI) images with a slice thickness of 5 mm for patients who underwent radical nephrectomy before surgery (A) delayed CT axial image (B) T2 weighted MRI axial image.
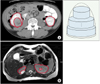
FIG. 2
Receiver operating characteristic (ROC) curve based on parenchymal volume of remnant kidney volume.
CI, confidence interval.

TABLE 1
Chronic kidney disease stage based on eGFR

Defined by the National Institute for Health and Clinical Excellence, 2008 [13].
eGFR, estimated glomerular filtration rate.
TABLE 3
Predictive factors for eGFR<60 mL/min/1.73 m2 5 years after radical nephrectomy (univariate and multivariate analysis)

References
1. Barlow LJ, Korets R, Laudano M, Benson M, McKiernan J. Predicting renal functional outcomes after surgery for renal cortical tumours: a multifactorial analysis. BJU Int. 2010. 106:489–492.
2. Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006. 7:735–740.
3. Jeon HG, Jeong IG, Lee JW, Lee SE, Lee E. Prognostic factors for chronic kidney disease after curative surgery in patients with small renal tumors. Urology. 2009. 74:1064–1068.
4. Suer E, Burgu B, Gokce MI, Turkolmez K, Beduk Y, Baltaci S. Comparison of radical and partial nephrectomy in terms of renal function: a retrospective cohort study. Scand J Urol Nephrol. 2011. 45:24–29.
5. Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Prim Care. 2008. 35:329–344. vii
6. Belldegrun A, Tsui KH, deKernion JB, Smith RB. Efficacy of nephron-sparing surgery for renal cell carcinoma: analysis based on the new 1997 tumor-node-metastasis staging system. J Clin Oncol. 1999. 17:2868–2875.
7. Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000. 163:442–445.
8. Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol. 2000. 163:730–736.
9. Simmons MN, Weight CJ, Gill IS. Laparoscopic radical versus partial nephrectomy for tumors >4 cm: intermediate-term oncologic and functional outcomes. Urology. 2009. 73:1077–1082.
10. Nouralizadeh A, Simforoosh N, Tabibi A, Basiri A, Ziaee SA, Soleimani M, et al. Laparoscopic partial nephrectomy for tumours >4 cm compared with smaller tumours: perioperative results. Int Urol Nephrol. 2011. 43:371–376.
11. Morrisroe SN, Su RR, Bae KT, Eisner BH, Hong C, Lahey S, et al. Differential renal function estimation using computerized tomography based renal parenchymal volume measurement. J Urol. 2010. 183:2289–2293.
12. Funahashi Y, Hattori R, Yamamoto T, Kamihira O, Sassa N, Gotoh M. Relationship between renal parenchymal volume and single kidney glomerular filtration rate before and after unilateral nephrectomy. Urology. 2011. 77:1404–1408.
13. National Institute for Health and Care Excellence. Chronic kidney disease: early identification and management of chronic kidney disease in adults in primary and secondary care. September 2008 [Internet]. c2013. cited 2013 Jan 10. London: National Institute for Health and Care Excellence;Available from: http://www.nice.org.uk/nicemedia/live/12069/42117/42117.pdf.
14. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004. 351:1296–1305.
15. Jeon HG, Gong IH, Hwang JH, Choi DK, Lee SR, Park DS. Prognostic significance of preoperative kidney volume for predicting renal function in renal cell carcinoma patients receiving a radical or partial nephrectomy. BJU Int. 2012. 109:1468–1473.
16. Sharma N, O'Hara J, Novick AC, Lieber M, Remer EM, Herts BR. Correlation between loss of renal function and loss of renal volume after partial nephrectomy for tumor in a solitary kidney. J Urol. 2008. 179:1284–1288.
17. Simmons MN, Fergany AF, Campbell SC. Effect of parenchymal volume preservation on kidney function after partial nephrectomy. J Urol. 2011. 186:405–410.
18. Jeon HG, Lee SR, Joo DJ, Oh YT, Kim MS, Kim YS, et al. Predictors of kidney volume change and delayed kidney function recovery after donor nephrectomy. J Urol. 2010. 184:1057–1063.
19. Gomez-Anson B, Carrero-Lopez V, Diaz-Gonzalez R. Image-directed color Doppler ultrasound evaluation of the single kidney after unilateral nephrectomy in adults. J Clin Ultrasound. 1997. 25:29–35.
20. Bakker J, Olree M, Kaatee R, de Lange EE, Moons KG, Beutler JJ, et al. Renal volume measurements: accuracy and repeatability of US compared with that of MR imaging. Radiology. 1999. 211:623–628.
21. Emamian SA, Nielsen MB, Pedersen JF, Ytte L. Kidney dimensions at sonography: correlation with age, sex, and habitus in 665 adult volunteers. AJR Am J Roentgenol. 1993. 160:83–86.
22. Mullerad M, Kastin A, Issaq E, Moskovitz B, Groshar D, Nativ O. The value of quantitative 99M technetium dimercaptosuccinic acid renal scintigraphy for predicting postoperative renal insufficiency in patients undergoing nephrectomy. J Urol. 2003. 169:24–27.
23. Ohno Y, Nakashima J, Ohori M, Hashimoto T, Iseki R, Hatano T, et al. Impact of tumor size on renal function and prediction of renalinsufficiency after radical nephrectomy in patients with renal cell carcinoma. J Urol. 2011. 186:1242–1246.
24. Funahashi Y, Hattori R, Yamamoto T, Kamihira O, Moriya Y, Gotoh M. Change in contralateral renal parenchymal volume 1 week after unilateral nephrectomy. Urology. 2009. 74:708–712.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download