Abstract
A 66-year-old man with a history of multiple transurethral resections for recurrent bladder tumors, staged as Ta according to the International Union Against Cancer staging guidelines, presented with a complaint of dry cough. A round nodule with a diameter of 7.5 cm was detected in the lung by chest computed tomography, and a video-assisted thoracoscopic lobectomy was performed. Pulmonary metastasis of recurrent bladder cancer was diagnosed by immunohistochemistry staining for the urothelium-specific protein uroplakin Ia. Subsequently, 2 cycles of systemic chemotherapy were administered. Two and a half years after treatment, no recurrence of pulmonary lesions has been detected. A combination of complete resection of pulmonary lesions and systemic chemotherapy may result in a good prognosis for patients with non-muscle-invasive bladder cancer.
Nonmuscle-invasive bladder cancer seldom shows distant metastasis. In addition, it is extremely rare for bladder cancer staged as Ta under the International Union Against Cancer guidelines to result in distant metastasis. We present the case of a patient with a distant pulmonary metastasis of Ta bladder cancer; the patient did not have a history of muscle-invasive disease. We review the literature concerning non-muscle-invasive bladder cancer with distant metastasis and discuss management and treatment outcomes.
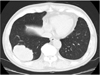
A 66-year-old man had been diagnosed with Ta bladder cancer 6 years before he complained of dry cough in July 2009. He had a history of recurrent non-muscle-invasive bladder tumors and had therefore undergone 7 transurethral resections (TURs) and had received intravesicular chemotherapies (8 cycles of weekly mitomycin-C after the 4th TUR and 5 cycles of biweekly pirarubicin after the 5th TUR). All tumors were <1 cm, well pedunculated, and papillary. The number of tumors ranged from 1 to 6 in each respective TUR. The pathological findings of all TURs indicated the presence of urothelial carcinoma (stage Ta without carcinoma in situ, grade 1 or 2) without lymphatic or vascular invasion. The last TUR in January 2009 showed the same findings as the previous TURs, i.e., there were 3 tiny papillary tumors located at the right lateral wall, at the posterior wall, and adjacent to the left ureteral orifice, and pathological examination indicated results similar to those of the previous TURs. Chest radiography at 6 months after the last TUR detected a round nodule (diameter, 7.5 cm) in the right lower lung. We carefully rechecked the chest radiograph obtained in April 2008, which showed a small nodule (diameter, 1 cm) in the same area. These observations suggest that the lung metastasis had occurred more than 1 year previously. The initial diagnosis based on the computed tomography findings was primary lung cancer (Fig. 1). Therefore, initial treatment consisted of video-assisted thoracoscopic lobectomy. The histopathologic features of the pulmonary lesion and the TUR specimen of the bladder cancer were qualitatively similar. Immunohistochemistry showed that both the TUR specimen and the pulmonary lesion were positive for the urothelium-specific protein uroplakin Ia (UP-Ia) [1] (Fig. 2). On the basis of these findings, the patient was diagnosed with pulmonary metastasis of recurrent Ta bladder cancer. Subsequently, he received 2 cycles of postoperative chemotherapy with the same gemcitabine and cisplatin regimen reported by von der Maase et al. [2]. Although we had planned 3 cycles of chemotherapy, the patient refused the third course. His bladder cancer recurred at 3 and 8 months after chemotherapy and hence he underwent TUR both times. The results of the pathological examination were the same results as for the previous TURs. Two and a half years after chemotherapy, chest and abdominal computed tomography once every 3 months detected no recurrence of the pulmonary lesions or other distant metastasis.
For nonmuscle-invasive bladder cancer, the probability of recurrence at 5 years ranges from 31% to 78% [3], whereas the risk of distant metastasis is extremely low. Matthew et al. [4] reviewed cases of more than 1,000 patients treated for bladder cancer, regardless of the stage, and identified 9 patients with superficial urothelial carcinoma and distant metastatic disease (including 3 patients with stage pTa disease). Three patients underwent radical cystectomy, and muscle invasion was not identified on pathological examination [4]. Because vessels are absent in the bladder mucosa, it is thought that Ta bladder cancer does not have the potential for lymphatic or hematogenous metastasis. Several iatrogenic mechanisms of metastasis in clinically diagnosed Ta cancer have been postulated: microscopic invasion by an understaged tumor, intravascular dissemination of tumor cells during transurethral resection, and degeneration of the basal membrane caused by intravesicular therapy [5].
It is often difficult to definitively diagnose pulmonary metastasis of bladder cancer. Histopathologic features of non-keratinized squamous-cell carcinoma of the lung are similar to those of urothelial carcinoma. Uroplakins (UPs) are urothelium-specific proteins and have 4 subtypes: Ia, Ib, II, and III. Kageyama et al. [1] reported that UP-Ia has higher sensitivity for urothelial carcinoma than UPs II and III. They investigated the expression of UP-Ia in primary and metastatic urothelial carcinoma. Among primary and metastatic lesions, the detection rate of UP-Ia was 96.8% and 72.2%, respectively. UP-Ia can be a promising marker for identifying urothelial carcinoma.
To the best of our knowledge, 15 cases of non-muscle-invasive bladder cancer with distant metastasis have been reported in the English literature. Metastatic sites include the lungs, liver, bones, ovaries, and brain. Furthermore, 7 cases of lung metastasis have been reported (Table 1). All patients with bone metastasis (including 2 with stage pTa disease) died shortly after diagnosis [4], and 1 patient with solitary brain metastasis died less than 3 months after brain surgery [6]. On the other hand, as indicated in Table 1, the outcomes in most patients with lung metastasis were good, except for 1 patient (stage Ta, grade 2) who died 1 month after diagnosis [4]. Other studies have demonstrated a good prognosis in 3 patients with Ta bladder cancer and lung metastasis after a combination of lobectomy and neoadjuvant or adjuvant chemotherapy [7-9]. Death due to pulmonary metastasis of Ta bladder cancer has not been reported, because the malignant potential of the metastatic lesions is low, and metastatic lesions can be completely resected with ease. Although the effects of adjuvant chemotherapy remain undetermined, some authors have reported that adjuvant chemotherapy may contribute to the removal of systemically circulating cancer cells.
A combination of complete resection of the lung lesions and systemic chemotherapy may lead to good prognosis for patients with non-muscle-invasive bladder cancer.
Figures and Tables
ACKNOWLEDGMENTS
The authors would like to express their gratitude to Dr. Susumu Kageyama, Department of Urology, Shiga University of Medical Science, for his support in supplying anti uroplakin Ia antibody and data evaluation.
References
1. Kageyama S, Yoshiki T, Isono T, Tanaka T, Kim CJ, Yuasa T, et al. High expression of human Uroplakin Ia in urinary bladder transitional cell carcinoma. Jpn J Cancer Res. 2002. 93:523–531.
2. von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000. 18:3068–3077.
3. Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006. 49:466–477.
4. Matthews PN, Madden M, Bidgood KA, Fisher C. The clinicopathological features of metastatic superficial papillary bladder cancer. J Urol. 1984. 132:904–906.
5. Kakehi Y, Nishio Y, Hashimura T, Takeuchi H, Yoshida O. Clinicopathological analysis on invasion and metastasis in superficial bladder cancer. Hinyokika Kiyo. 1992. 38:783–788.
6. Zennami K, Yamada Y, Nakamura K, Aoki S, Taki T, Honda N. Solitary brain metastasis from pT1, G3 bladder cancer. Int J Urol. 2008. 15:96–98.
7. Seymour JE, Malin JM Jr, Pierce JM Jr. Late metastases of a superficial transitional cell carcinoma of the bladder: report of a case. J Urol. 1972. 108:277–278.
8. Koh KB, Rogawski K, Smith PH. Cavitating pulmonary metastases from superficial transitional cell carcinoma of urinary bladder: case report. Scand J Urol Nephrol. 1994. 28:201–202.
9. Dougherty DW, Gonsorcik VK, Harpster LE, Trussell JC, Drabick JJ. Superficial bladder cancer metastatic to the lungs: Two case reports and review of the literature. Urology. 2009. 73:210e3–e5.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download